More Information
Submitted: February 26, 2024 | Approved: March 25, 2024 | Published: March 25, 2024
How to cite this article: Nada M, Rahma Y, Khouloud T, Faten G, Karima T, et al. The Accuracy of pHH3 in Meningioma Grading: A Single Institution Study. Arch Pathol Clin Res. 2024; 9: 006-011.
DOI: 10.29328/journal.apcr.1001041
Copyright License: © 2024 Nada M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Grade; Immunohistochemistry; Meningioma; Mitotic index; pHH3
Abbreviations: CNS: Central Nervous System; EDTA: Ethylenediaminetetraacetic Acid; ER2: Epitope Retrieval Solution 2; FFPE: Formalin-Fixed Paraffin-Embedded; HE: Hematoxylin and Eosin; HMPIT: Principal Military Hospital of Instruction of Tunis; HPF: High Power Fields; IHC: Immunohistochemistry; MI: Mitotic Index; MF: Mitotic Figure; pHH3: Phosphohistone H3; WHO: World Health Organization
The Accuracy of pHH3 in Meningioma Grading: A Single Institution Study
Mansouri Nada1, Yaiche Rahma1*  , Takout Khouloud1, Gargouri Faten1, Tlili Karima1, Rachdi Mohamed Amine2, Ammar Hichem2, Yedeas Dahmani2, Radhouane Khaled2, Chkili Ridha2, Msakni Issam1 and Laabidi Besma1
, Takout Khouloud1, Gargouri Faten1, Tlili Karima1, Rachdi Mohamed Amine2, Ammar Hichem2, Yedeas Dahmani2, Radhouane Khaled2, Chkili Ridha2, Msakni Issam1 and Laabidi Besma1
1Pathology Department, Military Hospital of Tunis, Montfleury, 1008, Tunis, Tunisia
2Neurosurgery Department, Military Hospital of Tunis, Montfleury, 1008, Tunis, Tunisia
*Address for Correspondence: Dr. Rahma Yaiche, MD, Pathology Department, Military Hospital of Tunis, Montfleury, 1008, Tunis, Tunisia, Email: [email protected]
Introduction: In the latest WHO classification of central nervous system tumors, Mitotic Index (MI) counted on Phosphohistone-H3 stained slides (pHH3-MI) has been suggested as a valid proliferative marker in various tumors including in the evaluation of meningioma grading.
We aim to report our own experience in assessing the efficiency of the anti-pHH3 antibody as a grading tool for meningiomas.
Methods: A retrospective study was conducted on a series of 40 meningiomas diagnosed from March 2020 to April 2021 at the Pathology Department of the Military Hospital of Tunis. We attempted immunohistochemistry and compared MI assessed on both pHH3 and HE-stained slides.
Results: According to the HE-MI and pHH3-MI, the 40 cases of meningiomas were respectively divided into 35 versus 29 grade 1 cases, four versus eight grade 2 cases, and one versus three grade 3 cases. A highly significant correlation was found between pHH3-MI and HE-MI (p < 0.001). A significantly higher sensitivity in the pHH3 counting method was reported in our study.
Discussion: we found, in accordance with the literature, that pHH3-MI is more reliable and accurate in mitotic counting, therefore exhibiting a high sensitivity in tumor grading, reported by an upgrade within 22,5% of the cases.
Conclusion: PHH3-MI count facilitated a rapid reliable grading of meningiomas. However, molecular characteristics that could have a potentially significant impact on tumor progression should be the subject of further research.
Meningiomas are mostly benign, slow-growing neoplasms, that originate from meningothelial cells within the meninges [1]. They are among the most prevalent primary Central Nervous System tumors (CNS), accounting for approximately one-third of all intracranial tumors [2]. Nevertheless, like other cancers, meningioma’s prognosis and inherent tendency to recur depend strongly on various histopathological parameters including the extent of resection, brain invasion, histologic grade, and especially tumor proliferation capacity [3]. Therefore, the 2021 revised fifth edition of the World Health Organization (WHO) classification system has adopted the Mitotic Index (MI) as a proliferation indicator. It represents the number of Mitotic Figures (MFs) per 10 consecutive High Power Fields (HPF), manually assessed on Hematoxylin and Eosin (HE)-stained slides. Based on the mitotic indices, the tumors are stratified into three prognostically significant grades with relatively increasing malignancy: grade 1, grade 2, and grade 3 [3,4].
The WHO Meningiomas’ grading system disclosed the limitation of accurately predicting the tumor’s behavior, founded on histomorphic criteria, which poses daily diagnostic challenges and appears to be time-consuming as well.
Besides, the mitosis assessment on HE specimens is inter-observer dependent, making it less reproductive as a tool. More includes the subjective determination of the highest mitotic activity area [5] and the difficulty in discriminating true mitotic figures from other resembling figures [2]. These factors and more may falsify the counts, contributing to inaccurate grades.
Therefore, ancillary techniques were required. Since immunostaining is a decent detection technique, several markers have been incorporated as research subjects. Immunohistochemistry using the Ki-67 labeling index was first attempted in guiding the assessment of mitotic count [1]. Although it was widely studied, nonetheless ki67 labeling index was subject to interlaboratory differences that affect staining and interpretation which results in less precise reflection of proliferating cells [6].
Other biomarkers have been assessed for a more exact evaluation of the proliferation index.
A specific mitosis-related protein called phosphohistone H3 (pHH3) is more and more studied as a proliferation marker in different types of tumors including meningiomas.
Herein, we attempted to investigate the effectiveness of the anti-pHH3 antibody in meningioma grading by comparing it to the standard mitotic count on HE slides.
Study type: We performed a retrospective study carried out over a 3-year period from March 2019 to April 2021.
Study sample: We included all patients (n = 40) who underwent surgery for an intracranial meningioma at the Department of Neurosurgery at the Principal Military Hospital of Instruction of Tunis (HMPIT) during our study period.
Inclusion criteria: Patients were operated for meningioma in the study period. Both first-diagnosed tumors and relapsed tumors were included.
Non-inclusion criteria: Patients operated for meningioma at an external institution or whose histological examination was performed at an external institution.
Exclusion criteria:
• Patients with incomplete or missing medical records.
• Patients whose tissue blocks were unusable or inadequate for an Immunohistochemistry (IHC) study.
Data selection
Clinical data: Patient records and anatomopathological examination applications were selected from the database system of the HMPIT.
Histological data: Material collection: The 40 cases of meningiomas were analyzed using two different methods: first, 40 standard Hematoxylin and Eosin (HE)-stained slides and paraffin-embedded tissue blocks were retrieved from the archive of the Pathology Department at the HMPIT and analyzed.
Then, from the same 40 paraffin blocks, new sections were made which were later used for immunohistochemistry (second method of analysis: section. 8).
Slides examination: All available HE-stained slides were reviewed by a pathologist.
Selected parameters: Based on the histological features, we recorded the following parameters for each case: age, gender, tumor location, histologic subtype, presence of psammoma bodies, atypia criteria, and mitotic indices.
Counting of mitosis on HE-stained slides
The mitotic indices were counted in HE-stained slides based on the number of MFs per 10 consecutive HPFs (x400 magnification) in the area of the highest mitotic activity as recommended in Perry’s study [4].
Immunohistochemistry technique
Antibody: We used a ready-to-use rabbit polyclonal anti-pHH3 antibody (keycode: CMC369000010, Catalog Number: 369A-1, dilution 1/100; 1.0 mL concentrate, ref.F/369A-17, from Cell Marque manufacturer, Rocklin, CA, USA).
Technique:
• Tissue preparation steps:
Antibody application areas were predefined by the same pathologist on the HE-stained slides.
Formalin-Fixed Paraffin-Embedded (FFPE) tissue specimens were first cut at 2 to 3 µm thickness using a microtome and then disposed of on salinized slides.
The slides were placed in a 60 °C oven to melt the paraffin and then conserved for 12 hours at 37 °C.
• IHC steps:
Immunohistochemistry was performed on the Leica Bond-max platform (Leica Biosystems Melbourne Pty Ltd, Australia, 2014).
After deparaffinization and rehydration, we proceeded to antigen unmasking: before processing the immunostaining, the antigen sites were revealed by breaking antigens cross-links. This step was performed using the EDTA-based pH 8,8 Epitope Retrieval solution 2 (ER2) at 100 °C for 20 min.
In order to block nonspecific bindings, tissue samples were pre-treated with a 3% hydrogen peroxide solution (H2O2) for 5 minutes to stop endogenous peroxidase activity.
The sections were incubated with the primary unlabeled anti-pHH3 antibody for 15 min. After that, a polymer-labeled secondary antibody was added and the slides were incubated for 8 min.
Then, the peroxidase activity was initiated by the addition of its DAB substrate as a chromogene in the presence of H2O2 for 10min, the latter was oxidized by the peroxidase resulting in unsoluble brown coloration.
Finally, sections were dehydrated by placing them in a series of alcohol of increasing concentrations (95%,100%). Mounting of slides was performed manually with coverslips using an aqueous Eukitt mounting medium (both an adhesive and tissue preservative).
After letting the medium set, the slides were ready to be analyzed by the pathologist.
Counting of mitosis on pHH3 stained slides
The 40 anti-pHH3-Immunostained prepared slides were evaluated by the same pathologist.
Like in HE-stained sections, pHH3-positive MFs were assessed in 10 consecutive HPFs (x400 magnification).
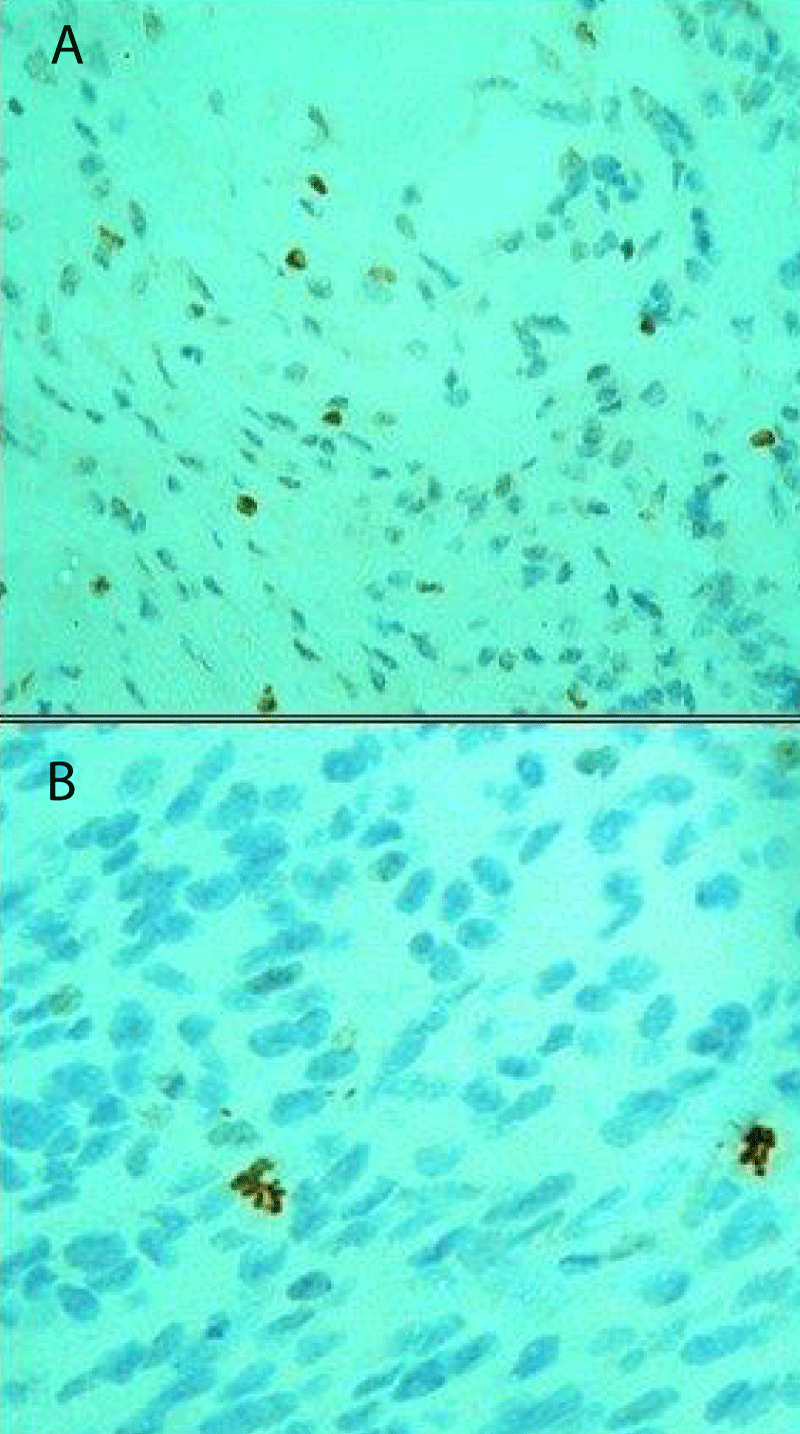
• Cells with a brown staining, non-conserved nuclear membrane, and presence of chromosome condensation were considered as positively stained MFs (Figure 1).
Figure 1: Examples of pHH3-positive stain (A) and pHH3 negative stain (B): A) True mitotic figures featuring brown color and absence of nuclear membrane (anti-pHH3 antibody immuno-stained slide x400). B) Mitotic nuclei feature a brown color however the nuclear membrane remains intact (anti-pHH3 antibody immuno-stained slide x400).
• Stained nuclei, with intact nuclear membranes and with no distinct chromosome condensation were not included (Puripat and Loharamtaweethong,2018) (Figure 2).
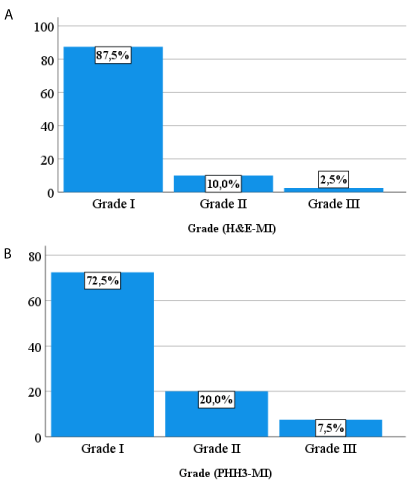
Figure 2: Histograms showing the different meningioma’s grading based on HE-MI (A) and pHH3-MI (B).
Statistical analysis
In order to establish a descriptive and analytic study, all files were filled up and entered into the SPSS Statistics 25 software. The correlation coefficient was calculated based on Pearson’s chi-square test correlation method, using the same software. The test is significant when the value is < 0.05.
Bibliographic research methods
We searched and gathered articles about meningioma and anti-pHH3 antibodies from bibliographic sources such as PubMed, NCBI, Springer, MDPI, and ELSEVIER, using the following keywords:
• Meningioma
• pHH3
• Histology
• Immunohistochemistry
• Mitotic index
• WHO classification
• Diagnosis
Study population
The cohort consisted of 24 females and 16 males with a sex ratio (male/female) = 2:3. Patients of the study were aged between 15 to 86 years, with a mean age of 59.53 years old. The study gathered a variety of locations with the most frequent ones being the frontal (17.5%) and parasagittal (12.5) locations.
Anatomopathological study
Histological types: Meningothelial and transitional types were predominant (50% versus 20%).
Mitotic count on Hematoxylin and Eosin stained slides: Meningioma cases were graded based singly on the number of mitotic figures (MFs) per 10 consecutive high-power fields (/10HPF) on Hematoxylin and Eosin-stained slides (HE-MI). The 40 cases were then divided into 35 grade 1 cases (less than 4 mitoses), 4 grade 2 (4 to 19 mitoses), and 1 grade 3 (20 or more mitoses) (Figure 2A).
Mitotic count on pHH3 slides: Following the same previous approach meningioma cases were graded based solely on the number of MFs per 10 consecutive HPFs on pHH3 immuno-stained slides (pHH3-MI). They grouped 29 grade 1 cases (less than 4 mitoses), 8 grade 2 (4 to 19 mitoses), and 3 grade 3 (20 or more mitoses) (Figure 2B).
Mitotic index and grade comparison
pHH3-MI was shown to be higher than the HE-MI in 47.5% of cases. The HE-MI and pHH3-MI mean values were compared for each grade as shown in Table 1.
| Table 1: Mean values of HE-MI and pHH3-MI for each grade. | ||||
| Grade 1 | Grade 2 | Grade 3 | p - value | |
| Mean HE-MI | 0,03 ± 0,17 (n = 35) | 5,75 ± 0,96 (n = 4) | 20 ± 0 (n = 1) | p < .001 |
| Mean pHH3-MI | 0,69 ± 1,14 (n = 29) | 6,75 ± 2,31 (n = 8) | 39,66 ± 34,06 (n = 3) | |
| *Values are expressed as mean ± standard deviation (SD). *p value significant < 0.05. |
||||
pHH3 mitosis counting led to a modification of the grade in 22,5% of the cases:
• 6 grade 1 meningiomas upgraded to grade 2 (atypical).
• Two grade 2 upgraded to grade 3 (anaplastic).
Correlation between HE-MF and pHH3-MF
The correlation between HE and pHH3 counting methods was high with a Pearson correlation coefficient: r = 0.954; p < .001.
In the following study, we performed Immunohistochemistry (IHC) on 40 meningioma cases to investigate the effectiveness of the anti-pHH3 antibody in meningioma grading.
Our study findings reported a significantly higher sensitivity in the pHH3 counting method, which resulted in an overall upgrading rate of 22,5%; 6 grade 1 meningiomas upgraded to grade 2, and two grade 2 cases upgraded to grade 3.
Epidemiologic data
Meningiomas are the most common intracranial primary tumors, representing 33.8% of all brain and central nervous system tumors [7].
Age: Meningiomas are more likely to occur with advancing age. They are most frequent between 60 and 70 years old [8]. The mean age for meningioma patients falls within the range of 57,3 to 61,3 [5, 9,10].
Our study series confirms the literature findings, with a mean age of 59.5 (range:15 years - 86 years old) supporting the increased incidence of meningioma in elderly individuals.
Gender: The meningeal tumor is more prevalent among women than men [5,11,12]. With a sex ratio (Male/Female) of 1:2 in a series of 45 cases reported by Fukushima, et al. [5] and even higher reaching 3:7 (following some order) within the series of 265 meningiomas studied by Kim, et al. [11].
In our present study, the sex ratio was slightly lower (2:3) than the previously introduced values; this may be due to a sampling bias due to the military status of our patients which may not represent the overall population.
Histologic type
Among all meningioma subtypes, the most frequently encountered are meningothelial, transitional, and Fibrous [5,12]. Within the series of Samadi and Ahmadi, et al. [12], they accounted for respectively 65,5%,17,2%, and 9,2%.
Our series conforms to the literature findings, with 50% meningothelial, 20% transitional, and 15% fibroblastic.
Mitotic count and grades
The Mitotic Index (MI) has been adopted by the World Health Organization (WHO) as the main criterion in meningioma grading. It is defined as the number of mitotic figures (MFs) per 10 consecutive high-power fields (HPFs), counted on Hematoxylin and eosin-stained slides [1]. Accordingly, the tumors are stratified into three grades: grade 1 (< 4 mitoses/10 HPFs), grade 2 (4 - 19 mitoses/10 HPFs), and grade 3 (≥ 20 mitoses/10 HPFs) [4].
Nevertheless, after being used for several years, the standard counting method revealed numerous limitations. The search for the highest mitotic activity area and the assessment of MFs are often difficult to accomplish [5], making the process both time-consuming and exhausting [11]. Besides, the counting method was shown to be inter-observer dependent, where values may fluctuate from one pathologist to another, resulting in controversies and less accurate diagnosis [5]. Furthermore, the occurrence of nuclear changes within apoptotic cells, crushed cells, or necrosis can easily mimic mitoses and therefore falsify the results [2].
Since the pHH3 was reported to be unique to mitosis, the anti-pHH3 antibody was being increasingly studied as a proliferation marker [5,9,11].
The evaluation of mitotic figures on the pHH3-immunostained slides was shown to be less tedious and much easier to approach than the standard HE staining method. It was also easy to discriminate true mitotic figures from other resembling figures owing to the specificity of pHH3 to mitosis. The pHH3 counting method minimized the unwanted deceptive elements and improved the clarity of MFs, which makes it much of a straightforward process. These outcomes conform to the literature findings [5,11].
The comparison of the mean values of MI assessed on both pHH3-stained slides (pHH3-MI) and HE Stained slides (HE-MI) revealed that the (pHH3-MI) tended to be superior in all three grades. Based on the previous statements, these results were highly predicted; a more reliable counting method may result in more significant and accurate mitotic indices. Similar results were reported by previous research [5,13], as presented in Table 2.
| Table 2: Comparison of mean values of HE-MI and pHH3-MI for three studies. | ||||||
| Grade 1 | Grade 2 | Grade 3 | ||||
| HE-MI | pHH3-MI | HE-MI | pHH3-MI | HE-MI | pHH3-MI | |
| - Ribalta, et al. [13] | 1.4 | 2,2 | 9,0 | 15,9 | 22,4 | 34,1 |
| - Fukushima, et al. [5] | 0,95 | 1,43 | 6,71 | 8,43 | 32 | 39 |
| - Our study | 0,02 | 0,68 | 5,75 | 6,75 | 20 | 39,66 |
| *For each grade we presented the mean of MI per 10 HPFs. | ||||||
Accordingly, regarding meningiomas based on the pHH3-MI induced an increase in grade accounted for 17% within the series of Ribalta, et al. [13] and 10,5% in the series of Kim, et al. (10); 27 grade 1 cases upgraded to grade 2 and one grade 2 upgraded to grade 3. Our results also confirm the literature, with an overall upgrading rate of 22,5% in all cases (6 grade 1 meningiomas upgraded to grade 2, and two grade 2 upgraded to grade 3).
Highlighting the fact that MI and the tumor grades move in the same direction, a higher MI value may potentially upgrade the tumor while lower values may downgrade them. Consequently, since the pHH3-MI showed to be superior to the HE-MI, an increase in grade was expected to ensure the high sensitivity of the pHH3 counting method [11].
Correlation
The linear regression analyses indicated a statistically significant correlation between HE-MI and pHH3-MI [9,11] (the values of pHH3-MI increase as the values of HE-MI increase). Based on the mitotic counts provided by rater 1 (senior 1), the Pearson correlation coefficient was found to be r = 0,92; p < .001 [9]. Comparably, Kim, et al. [11] reported similar outcomes with r = 0,9; p < .00001 [11]. Our study confirms the literature findings, with Pearson’s r = 0.954; p < .001.
A significant correlation indicates that the anti-pHH3 antibody counting method and the standard method are trending the same way, which conforms to our previous outcomes. The correlation confirms that the pHH3 counting method does not refute the HE method. Instead, it compensates for the standard method limitations through necessary corrections.
Considering the sensitivity of the pHH3 marker in MFs recognition, it is evident that the anti-pHH3 antibody might be a reliable tool for meningioma grading [11]. As a result, it can guarantee a more accurate cancer prognostic and management.
Study limitations
The results of this retrospective study have to be seen in the light of some limitations:
- Sample size: Our study included only 40 cases making it less representative of a population.
- Lack of data: Among the studied cases, 15% had no available data related to tumor location.
- We used polyclonal anti-pHH3-antibody which can bind to different epitopes of a single antigen instead of a monoclonal antibody that can only bind to one specific epitope of a single antigen and thus, be more specific.
The histological grade is the main prognostic indicator of meningioma. It depends mainly on the mitotic index. Mitotic count via pHH3 is not included in meningioma grading. Our study showed that this method was more accurate to establish the mitotic count since the mitotic index was higher in pHH3 stained slides than in HE stained slides and thus, resulted in upgrading in most of the meningiomas.
With the recent development of the molecular field, it would be interesting to explore molecular alterations that may have a considerable impact on the tumor behavior such as the status of pTERT mutation in cases of meningiomas with upgrade.
We would like to thank the staff of the departments of pathology and neurosurgery for their substantial contributions in developing the research idea: MN conceived and wrote the presented idea. YR handled the drafting of the work, its revision, and the submission process. TK performed the immunohistochemistry. GF proceeded to the mitotic counting on pHH3 slides. TK Proceeded to the mitotic counting on HE slides. RMA collected the clinical data. YD designed the figures and wrote the captions. AH and KR collected the references. CR, MI, and LB verified the final version of the manuscript.
Data availability statement: Data will be available on request.
Ethical consideration
• Approval of the research protocol: The study protocol was approved by the Ethics Committee of HMPIT, Tunis, Tunisia. During our study, we ensured full respect for the fundamental ethics of medical research:The principle of general interest and research beneficence
• Respect for the privacy and confidentiality of patient data
• The principle of non-maleficence
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021 Aug 2;23(8):1231-1251. doi: 10.1093/neuonc/noab106. PMID: 34185076; PMCID: PMC8328013.
- Puripat N, Loharamtaweethong K. Phosphohistone H3 (PHH3) as a surrogate of mitotic figure count for grading in meningiomas: a comparison of PHH3 (S10) versus PHH3 (S28) antibodies. Virchows Arch. 2019 Jan;474(1):87-96. doi: 10.1007/s00428-018-2458-2. Epub 2018 Sep 29. PMID: 30267302.
- Saffar H, Okhovat H, Arbabsoleymani S, Tavangar SM, Khoshnevisan A, Hajinasrollah G, Hamidi Afra Z, Saffar H. The Utility of Phosphohistone H3 in Inter-Observer Variability of Mitotic Count in Meningioma, is There Any Benefit? Asian Pac J Cancer Prev. 2021 Jul 1;22(7):2049-2052. doi: 10.31557/APJCP.2021.22.7.2049. PMID: 34319026; PMCID: PMC8607091.
- Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997 Dec;21(12):1455-65. doi: 10.1097/00000478-199712000-00008. PMID: 9414189.
- Fukushima S, Terasaki M, Sakata K, Miyagi N, Kato S, Sugita Y, Shigemori M. Sensitivity and usefulness of anti-phosphohistone-H3 antibody immunostaining for counting mitotic figures in meningioma cases. Brain Tumor Pathol. 2009;26(2):51-7. doi: 10.1007/s10014-009-0249-9. Epub 2009 Oct 27. PMID: 19856215.
- Elmaci İ, Altinoz MA, Sari R, Bolukbasi FH. Phosphorylated Histone H3 (PHH3) as a Novel Cell Proliferation Marker and Prognosticator for Meningeal Tumors: A Short Review. Appl Immunohistochem Mol Morphol. 2018 Oct;26(9):627-631. doi: 10.1097/PAI.0000000000000499. PMID: 28777144.
- Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010 Sep;99(3):307-14. doi: 10.1007/s11060-010-0386-3. Epub 2010 Sep 7. PMID: 20821343; PMCID: PMC2945461.
- Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, Vecht C. Meningioma. Crit Rev Oncol Hematol. 2008 Aug;67(2):153-71. doi: 10.1016/j.critrevonc.2008.01.010. Epub 2008 Mar 14. PMID: 18342535.
- Duregon E, Cassenti A, Pittaro A, Ventura L, Senetta R, Rudà R, Cassoni P. Better see to better agree: phosphohistone H3 increases interobserver agreement in mitotic count for meningioma grading and imposes new specific thresholds. Neuro Oncol. 2015 May;17(5):663-9. doi: 10.1093/neuonc/nov002. Epub 2015 Feb 1. PMID: 25646026; PMCID: PMC4482862.
- Ozek E, Akdag H, Tosuner Z, Abdallah A, Hatiboglu MA. The correlation between phosphorylated Histone H3 (PHH3) and p-STAT3 in Meningiomas. Clin Neurol Neurosurg. 2019 Mar;178:46-50. doi: 10.1016/j.clineuro.2019.01.016. Epub 2019 Jan 25. PMID: 30710729.
- Kim YJ, Ketter R, Steudel WI, Feiden W. Prognostic significance of the mitotic index using the mitosis marker anti-phosphohistone H3 in meningiomas. Am J Clin Pathol. 2007 Jul;128(1):118-25. doi: 10.1309/HXUNAG34B3CEFDU8. PMID: 17580279.
- Samadi N, Ahmadi SA. Meningioma: a clinicopathological evaluation. Malays J Med Sci. 2007 Jan;14(1):46-52. PMID: 22593651; PMCID: PMC3351217.
- Ribalta T, McCutcheon IE, Aldape KD, Bruner JM, Fuller GN. The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteria. Am J Surg Pathol. 2004 Nov;28(11):1532-6. doi: 10.1097/01.pas.0000141389.06925.d5. PMID: 15489659.