More Information
Submitted: October 05, 2023 | Approved: October 23, 2023 | Published: October 25, 2023
How to cite this article: Tena-Suck ML, Macias LC, Gómez-Apo E, Plata AO, Rubio C. Expression of Collagen VI, Anticollagenase, Laminin, MM9, Claudins 1 and 5, N and E Cadherins in Choroid Plexus Tumors. Arch Pathol Clin Res. 2023; 7: 020-027.
DOI: 10.29328/journal.apcr.1001037
Copyright License: © 2023 Tena-Suck ML, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Choroid plexus; Choroid plexus tumors; Extracellular matrix; Collagen IV; Claudins 1 and 5; Immunohistochemistry
Abbreviations: CP: Choroid Plexus; CPECs: Choroid Plexus Epithelial Cells; CSF: Cerebrospinal Fluid; WHO: World Health Organization; CPTs: Choroid Plexus Tumors; CPP: Choroid Plexus Papillomas; ACPP: Atypical Choroid Plexus Papillomas; CPC: Choroid Plexus Carcinomas; TJ: Tight Junction; JAM: Junction Adhesion Molecules; PFS: Progression-Free Survival; OS: Overall Survival; BBB: Blood-Brain Barrier; EMT: Epithelial-to-Mesenchymal Transition; SIRS: Systemic Inflammatory Response Syndrome; ECM: Extracellular Matrix
Expression of Collagen VI, Anticollagenase, Laminin, MM9, Claudins 1 and 5, N and E Cadherins in Choroid Plexus Tumors
Martha Lilia Tena-Suck1, Laura Chavez Macias2, Erick Gómez-Apo2, Alma Ortiz Plata1 and Carmen Rubio3*
1Department of Neuropathology, National Institute of Neurology and Neurosurgery, México City, Mexico
2Department of Pathology, General Hospital of México City, Mexico
3Department of Neurophysiology, National Institute of Neurology and Neurosurgery, México City, Mexico
*Address for Correspondence: Carmen Rubio, PhD, Department of Neurophysiology, National Institute of Neurology and Neurosurgery, Manuel Velasco Suárez”, Av. Insurgentes Sur No. 3788 Col. La Fama. Delegación Tlalpan C.P. 14269, México, DF, México, Email: [email protected]
Background: CPTs are rare intraventricular papillary neoplasms derived from the choroid plexus epithelium. Anti-collagenase and extracellular matrix which have not been expressed in brain tumors.
Objective: The purpose of this study was to investigate the expression levels of collagen type VI, anti-collagenase, laminin, MM9, claudins 1 and 5, N and E cadherins, and collagen VII, tejido, and collagen degradation enzyme complexes in choroid plexus tumors.
Materials and methods: We studied the expression of adhesion molecules, extracellular matrix, and anticollagenase with an immunohistochemistry approach and electron microscopy analysis in 42 choroid plexus tumors.
Results: 28(67%) were choroid plexus papillomas, 8 (19%) were atypical choroid plexus papillomas and 6 (14%) were choroid plexus carcinomas. The Ki67-li and MVD increased from CPC to ACPP, being the highest in malignant tumors as well as a strong immunoexpression of anti-collagenase and were inverse correlation with claudin 5, E, and N cadherin and collagen IV immunoexpressions which added further significant information to the prognosis and varied according to the histologic classification. By ultrastructure, the loss of basal membrane and cilia, disorganization, and proliferation of ECM were observed in CPC. Cerebral homeostasis largely results from the ability of both the Blood–Brain Barrier (BBB) at the brain microvascular endothelium and the Blood–Cerebrospinal Fluid Barrier (BCSFB) at the epithelium of the Choroid Plexuses (CPs), to control the composition of the CSF and cerebral extracellular fluid. Under expression of the tight junction proteins occludin, claudin-1 and claudin-5 are key molecular abnormalities responsible for the increased permeability of tumor endothelial tight junctions.
Conclusion: The loss of basement membrane and ECM overexpression could be considered as a poor prognosis predictor in CPT. Anti-collagenase and MMP9 overexpression could be related to basal membrane and BBB plasticity in CPTs.
The Choroid Plexus (CP) is located in the cerebral ventricles and is highly vascularized tissue, in which blood microvessels are enclosed by a single layer of cuboid epithelial cells [1]. It consists of epithelial cells, fenestrated blood vessels, and the stroma, dependent on various physiological or pathological conditions, which may contain fibroblasts, mast cells, macrophages, granulocytes, or other infiltrates, and a rich ECM [1].
Choroid Plexus Epithelial Cells (CPECs) are closely connected to each other by tight junctions and constitute the structural basis of the blood-CSF barrier [2]. The barrier function can be subjected to modulation and thereby regulates the entry of physiologically important substances [1-3]. Several peptides have been shown to be actively transported by the CPECs to the CSF and most of the transported hormones evidently have, at least, a hypothalamic destination [4].
The CPECs are involved in a variety of neurological disorders, including neurodegenerative, inflammatory, infectious, traumatic, neoplastic, and systemic diseases. According to the current 2016 World Health Organization (WHO) classification scheme [5], human Choroid plexus tumors (CPTs) are classified into 3 different grades: choroid plexus papillomas (CPP, grade I), atypical choroid plexus papillomas (ACPP, grade II), and choroid plexus carcinomas (CPC, grade III) [5]. Increasing tumor grade correlates inversely with survival time after surgery and is therefore an important prognostic factor [5].
Extracellular Matrix (ECM) is highly dynamic as it is constantly deposited, modified, and degraded during development until maturity to maintain tissue homeostasis. ECM is a physiologically active element of living tissue, controlling cell–cell communication, adhesion and proliferation, tumor invasion, metastasis, and microenvironment [6].
Expression of cell adhesion molecules [7] and Tight Junction (TJ) proteins that link the cells forming theseblood–brain interfaces form the anatomical basis for this control by preventing non-specific paracellular leakage between blood and the cerebral fluids [8,9]. Adherens junctions are composed of a cadherin–catenin complex and its associated proteins [8]. The TJ consists of three integral membrane proteins, namely, claudin, occludin, and Junction Adhesion Molecules (JAM), and a number of cytoplasmic accessory proteins including ZO-1, ZO-2, ZO-3, cingulin, and others [8-10]. Claudins are tight junction membrane proteins that are expressed in epithelia and endothelia and form paracellular barriers and pores that determine tight junction permeability [11].
The aim of this article was to find a correlation between the immunoexpression of immunomarker for ECM and tight junctions in choroid plexus tumors using immunohistochemistry, and ultrastructural approaches.
Criteria for patient selection
Our research procedures were conducted in adherence to the ethical guidelines set forth by the National and Institutional Research Committees, the 1964 Declaration of Helsinki, and any subsequent amendments to that document, or comparable standards of ethics. Hospital Ethics Committee and the Ethics Committee of the National Institute of Neurology and Neurosurgery (protocol number: 0291-M1-20 on 10 April 2015). Prior to participating in the investigation, all subjects provided informed consent.
42 primary and recurrent choroid plexus tumors were retrieved from the files of the Department of Neuropathology identified for entry into pathology archives operated from 1990 to 2009 at the National Institute of Neurology and Neurosurgery (INNN) in México City. 28(67%) were CPP, 8 (19%) were ACPP and 6 (14%) were CPC.
Clinical information and follow-up data were available in all cases and were graded according to the WHO classification [5]. Clinical data analyzed were: Age, gender, localization, size of tumors, time of onset of symptoms, surgical exeresis, recurrence, and Progression-Free Survival (PFS) were recorded. Data on age at diagnosis, degree of surgical resection, further treatment, and current status were obtained to assess prognostic implications of the current grading system, of histological and immunohistochemical features.
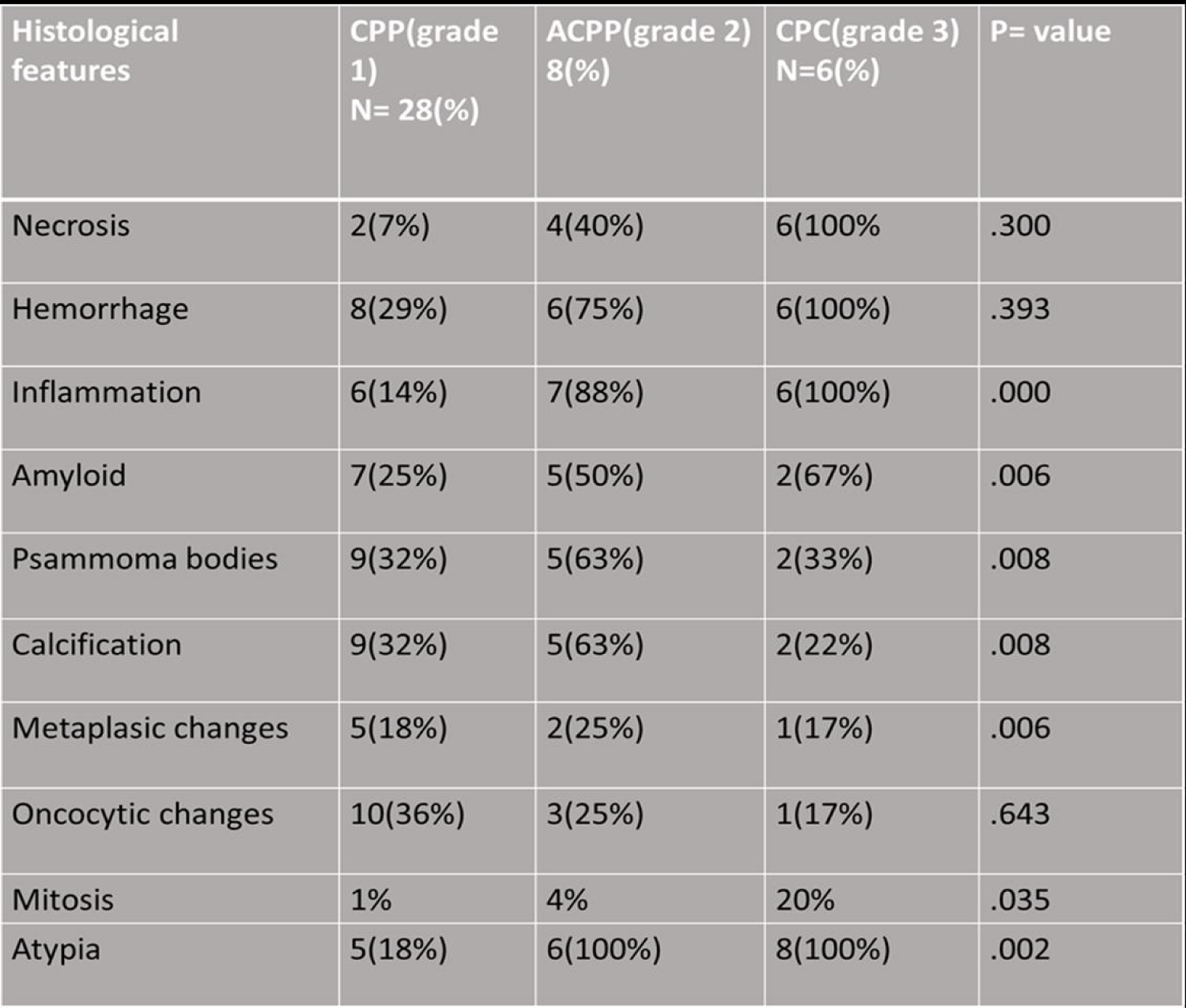
All tumors were formalin-fixed (10%) and paraffin-embedded. Sections were cut at 5 µm and stained with hematoxylin and eosin. In addition, the following criteria were assessed: evidence of peripheral capillary proliferation infiltrates with mononuclear inflammatory cells, chronic Inflammation (presence of lymphocytes, plasma cells infiltration), mineralization, gross and hyalinized vessels, and macrophages, necrosis, hemorrhage, pleomorphism, atypia, mitotic index, mineralization, psammoma bodies, brain infiltration, and gliosis were evaluated in each case. Afterwards, we performed a clinicopathological correlation.
Immunohistochemistry
All CPTs and NCPs were investigated with immuno-histochemistry using the following primary antibodies; Collagen IV (Collagen IV Antibody Monoclonal (CIV 22) (MA5-13437. Dilution 1:100), anti collagenase (Anti-Collagenenase antibody (ab6586) | Abcam, dilution 1:100), Vimentin antibody (E-5) SCBT | Santa Cruz Biotechnology, dilution 1:100), MMP9 (Anti-MMP9 antibody (ab38898) | Abcam). Laminin (Laminin alpha 5 Antibody (CL3118) (NBP2-42391): Novus, Dilution 1:50), Claudin 5 (claudin-5 Antibody (H-52): sc-28670 - Santa Cruz, Dallas, TX, dilution 1:100), Claudin 1(claudin-1 Antibody (C-18): sc-17658 - Santa Cruz, Dallas Tx, dilution 1:100), E-cadherin (E-cadherin Antibody (H-108): sc-7870 - Santa Cruz, Dallas Tx, Dilution 1:100) and N-cadherin (N-cadherin Antibody Santa Cruz Biotech (K-20), Dallas Tx, dilution 1:100) and Ki67(clone mib1, Biocare, dilution 1:100) Sections were deparaffinized in xylene and rehydrated in 100% ethanol. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide in methanol. The antigen was then damasked by heat retrieval in citrate buffer (pH 6.0) at 99 °C for 20 min using a laboratory microwave. Tissue sections were then blocked with 5% normal goat serum for 30 min. The sections were then incubated with the primary antibodies either overnight at 4 °C. Subsequent steps were performed with a commercial kit and according to the manufacturer’s instructions. For negative controls, the primary antibody was replaced by unspecific immunoglobulin G from nonimmunized mice or rabbits, as appropriate. Additionally, the intensity of the staining was assessed semi-quantitatively (0 – 3), and the staining pattern (membranous, cytoplasmic, nuclear) was determined. On the basis of the percentage of positive cells, each tumor was defined within 1 of 4 groups as follows: negative, < 30%, 30% - 60%, or > 60% positive.
Ki-67 labeling index was also performed. The MIB-1 labeling index was calculated as the percentage of positively stained tumor cell nuclei among 1000 nuclei detected in areas with the greatest degree of immunostaining. The mean, median, and Standard deviation of they were used as a reference to establish a cut-off point [12].
Ultrastructural analysis
From the paraffin blocks, tissue was obtained, included in glutaraldehyde, and processed for electron microscopy.
Statistical analysis
The statistical analysis was performed with statistical software (SSPS20). Mean and SD were evaluated. Continuous data were compared with the Mann–Whitney rank-sum test and categorical data with the Fisher exact test. p ≤ 0.05 was considered significant.
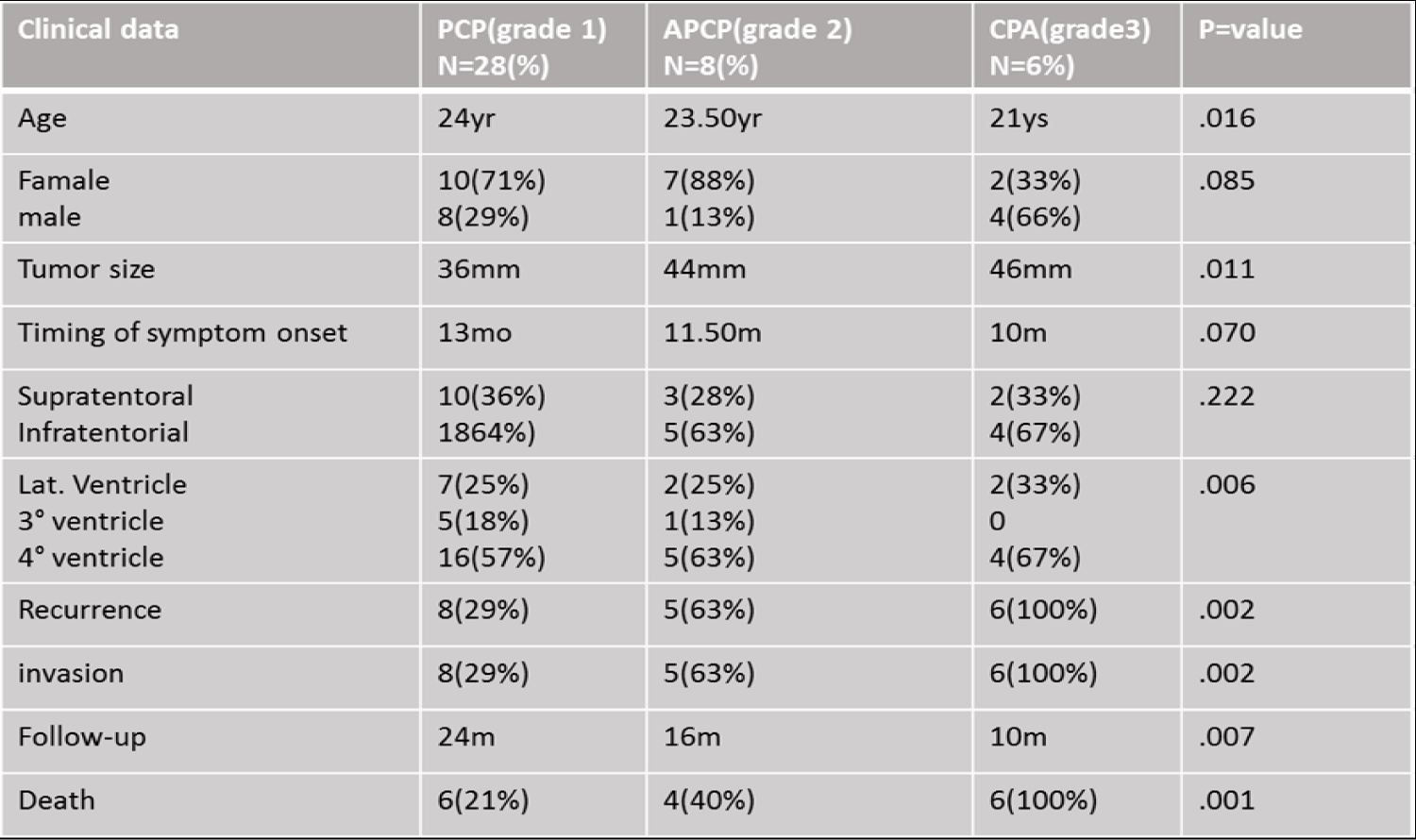
Forty-two CPTs were included in this study, from which 28(47%) were choroid plexus papilloma grade I, 8 (13%) were atypical choroid plexus papilloma grade II, and 6 (10%) were choroid plexus carcinomas grade II according to the WHO classification [4]. Table 1 shows the clinical characteristics of the patients, 27 were female (64%), and 15 were males (36%). Tumor localization; supratentorial portion were 15(36%), and infratentorial region in 27(64%), 11(18%) were located in lateral ventricles, 6(10%) in III ventricle and 25(42%) in IV ventricle. The range of age was from 17 to 67 years (mean of 28.63 ± 1.522), the mean age for females was 41.42 ± 1.465 and for males was 45.39 ± 1.769ys (Table 1).
Table 1: Showed the clinical and epidemiological study of the patients.
The tumor size varied from 24 to 65 mm (average of 43.07 ± 1.45) and the time of symptom onset was from 8 to 25 months (mean of 15.93 ± .685mo), the mean for females was 14.74 ± .767 and for males was 17.87 ± 1.208 mm.
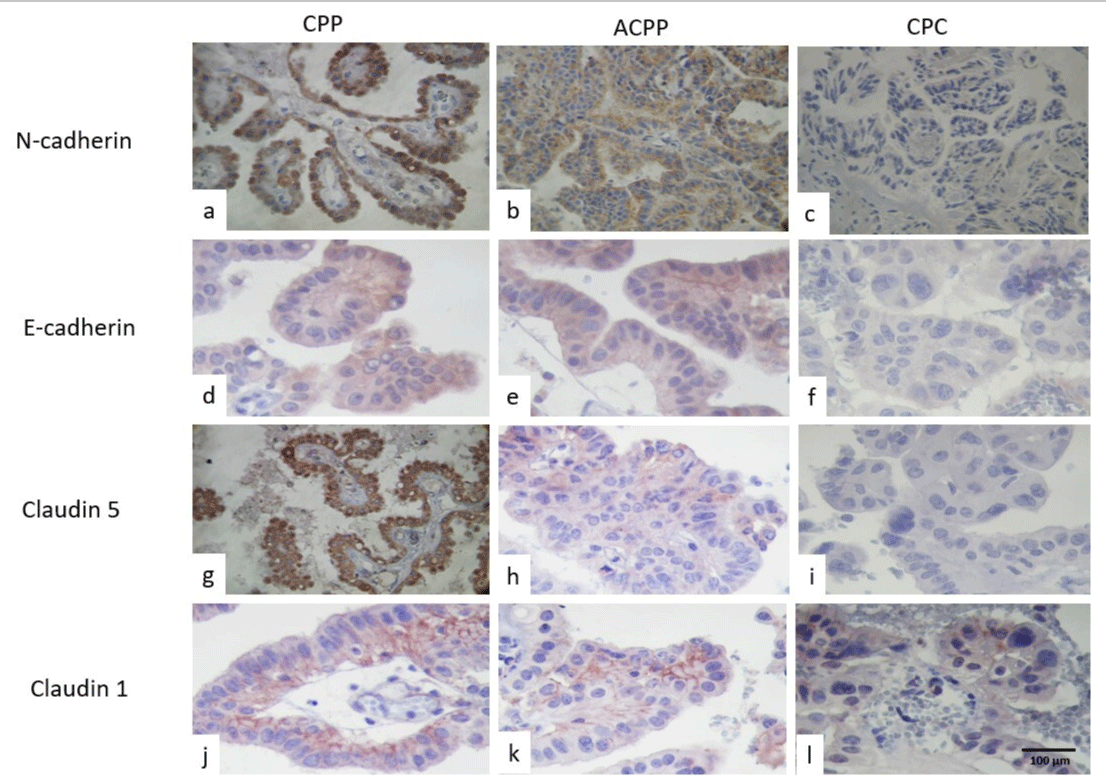
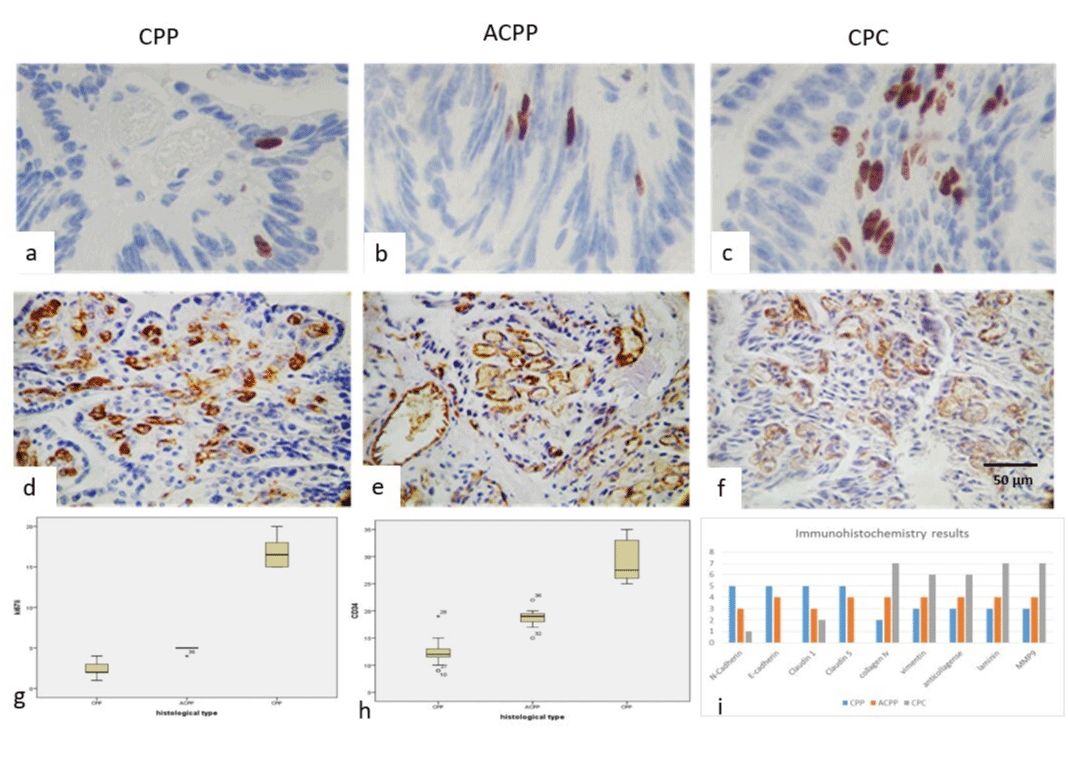
Immunohistochemistry results are seen in Table 2. N-cadherin was positive in both tumors, with stronger immunoreaction in CPC than CPP (Figures 1a-1c), however, E-cadherin was strongly positive in CPP than in CPC (Figures 1d-1f). Claudin 5 (Figures 1g-1i) was positive in CPP and negative in CPC, and finally claudin 1 had strong positive immunoreactions in CPP and negative in the epithelial cells in CPC, but it was only positive in the basal membrane in ACPP (Figures 1j-1l).
Table 2: Showed the histological features on the choroid plexus tumors.
Figure 1: Showed the immunoexpression of Cadherin in CPP(a), ACPP in (b), and CPC in (c). E-cadherin immunoexpression was higher in CPP (d), weak in ACPP (e), and negative in PCC (f). Claudin 5 was positive in CPP (g) and in ACPP (h) and negative in CPC (i). Claudin 1 was positive in the basal membrane of epithelial cells in CPP (j) and in ACPP(k) and was negative vs low and focal in CPC(h) (IHQ stain, original magnification x400).
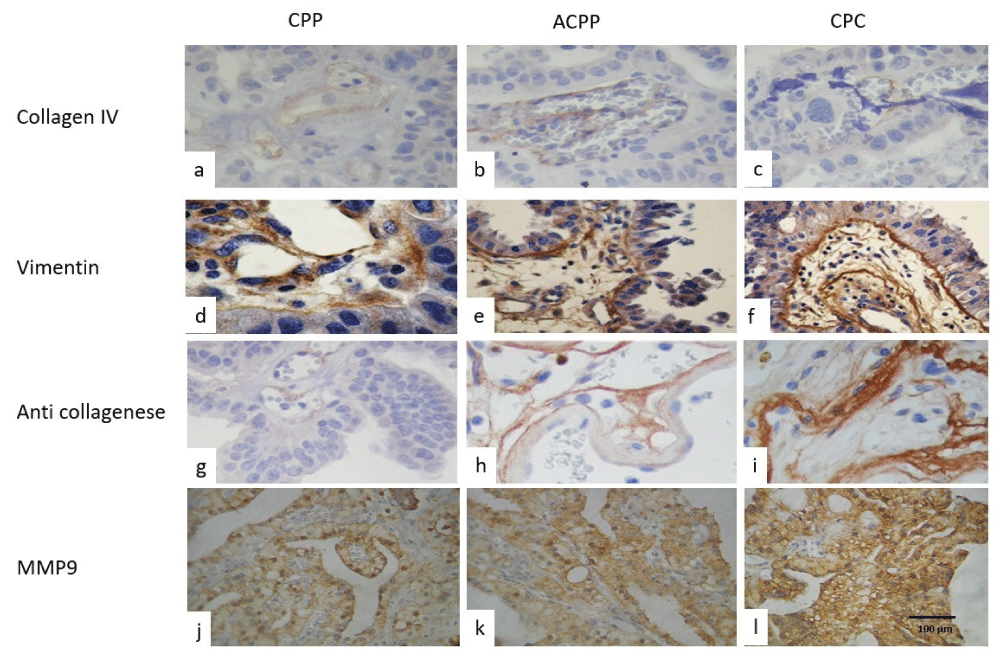
Collagen IV showed a weakly positive immunoreaction in the blood vessel walls, but with an important loss of the expression in CPCs than in CPP, in both CPTs. Vimentin was positive in the blood vessel walls, as well as in the internal basal membrane, and weak positive immunoreaction in epithelial cells cytoplasm in CPC (Figures 2d, -2f). Anti-collagenase was positive in the basal membrane and in the external border of epithelial cells, displaying a greater intensity in CPP and loss of epithelial expression and was more intense immunoreaction in the stroma of papillae in CPC (Figures 2g-2i). Laminin, and MMP9 (Figures 2j-2l) were also positive in the basal membrane and in the external border of epithelial cells more intense in CPP and loss of epithelial expression and was more intense immunoreaction in the stroma of papillae in CPC.
Figure 2: Showed positive immunoexpression of Collagen IV in the stroma and perivascular distribution, was strong in (a) CPP, (b) weak in ACPP, and focal or negative in CPC (c), Vimentin immunoexpression was positive in CPP (d), in ACPP (e), and in strong in PCC (f). while anti-collagenase was the focal and perivascular location in CPP (g), and in ACPP (h) and strong fibers positive in CPC (i). As well as, MM9 was positive in the basal membrane of epithelial cells and in the stroma in CPP (j) and in ACPP(k) and was stronger and diffused in CPC (h) (IHQ stain, original magnification x400).
The mean value of the Ki67-li was 3% for CPP, 6% for ACPP, and 13 for CPC. Ki67 labeling index was also higher in CPC than CPP (Figures 3a-3c), CD34 was positive in the wall of the blood vessels and was higher in CPC (features 3d, 3e and 3f). The mean value for MVD was 10% for CPP, 15 for ACPP, and 20% for CPC. The box plot of Ki67 is seen in (Figure 3g). CD34 and histological grade are seen in (Figure 3h) The histogram of the immunohistochemical results is seen in Figure 3i).
Figure 3: Immunoexpression of Ki67 was low in CPP (a), with discrete augmentation in ACPP (b) and higher in CPC (c), as well as the microvascular labeling index with CD34 immunoexpression was low in CPP (d), weak in ACPP (e), and high in PCC (f). (IHQ stain, original magnification x400). (g) Showed box and whiskers of ki67 in relation to histological grade and in (h) MVD box and whiskers in relation to histological grade and in (i) observed the different primary antibodies used in relation to histological grade.
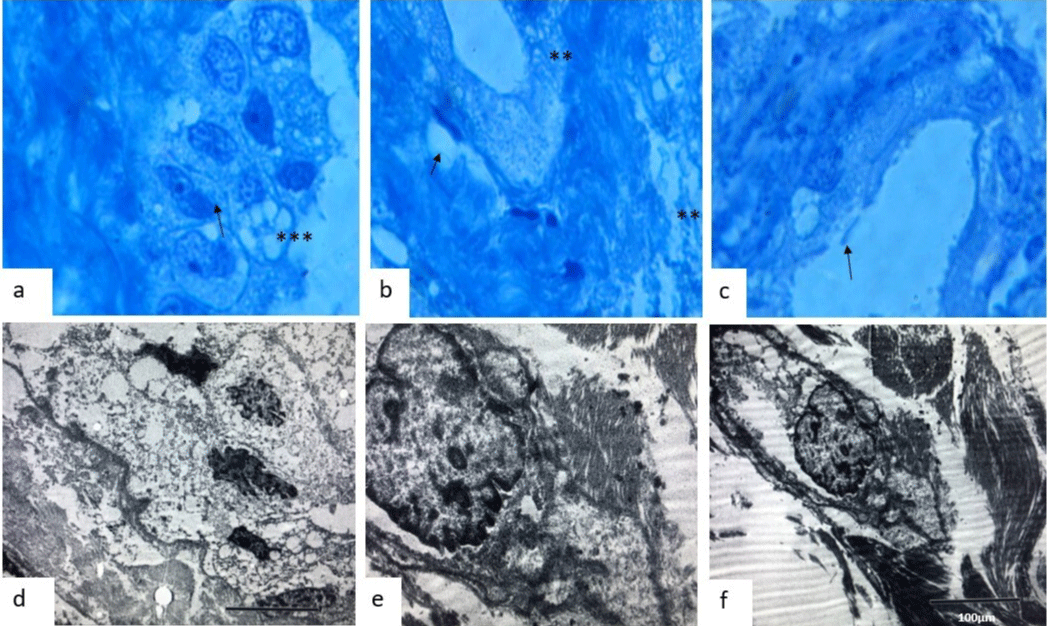
In semifinal sections, we observed that CPP has an apparently conserved basement membrane, and in between the intercellular junctions, we observed a loss of continuity, getting weaker (Figures 4a-4c, with intracytoplasmic vesicle formation and stromal fiber proliferation over the fibrovascular core stroma (Figure 4b-4c). In electron microscopy we observed a complete loss of epithelial cell polarity (Figure 4d), with nuclear pores and intracytoplasmic microtubule redistribution (Figure 4e), with the vacuolar formation and loss of basement membrane (Figure 4f), loss and cluster formation of cilia, some cells display anomalous desmosomes and reinforced/fragmented intercellular junctions. In the fibrovascular core, we observed the proliferation of fibrils and we even observed alteration in the normal structure of CPC cells.
Figure 4: Semifine courts with toluidine blue stain showed (a), (b), and (c) loss of cilia (asteristic), and (b) showed loss of basal membrane (arrows)(x100). (d) Electron microscopic feature which showed an irregularity in the nuclear membrane in CPC (x2000). (e) Observed the irregularities of the desmosomes and intercellular unions (x500) and (f) observed intracellular matrix and fragmentation of intermediate filaments in the fibrovascular core. We observed the proliferation of fibrils and we even observed alteration in the normal structure of CPC cells (x5000).
The Choroid Plexus Epithelial Cell (CPEC) is a complex epithelial-endothelial composed of an epithelium, stroma, and vascular supply. The stroma contains fibroblasts, inflammatory cells, and a rich extracellular matrix [13]. The Choroid Plexus (CP) is formed by ciliated columnar epithelial cells that secrete a large, uncharacterized, number of bioactive factors apically into the cerebrospinal fluid and basally into the circulation and produce and enrich cerebrospinal fluid in the adult and transport and secretory functions in CP support CSF homeostasis and fluid dynamics essential for brain function. CP of the Blood-CSF Barrier (BCSFB) displays fundamentally different properties than the blood-brain barrier (BBB) [1-3].
The CPEC morphology is balloon-shaped rather than cuboidal, and the microvilli of the apical surface of the CP epithelium contain more vacuoles [1]. Transcellular epithelial active transport and secretion are energized and channeled via a highly dense organelle network of mitochondria, endoplasmic reticulum, and Golgi, a fine basal membrane and incomplete tight junctions and low-capacity transcellular pinocytosis/exocytosis [6].
Different molecular factors involved in choroid plexus tumor biology may aid in identifying patients at risk for recurrence: like tight junction tumors, tight cell-cell associations known as adherent’s junctions, extracellular matrix, and basal membrane have been implicated in blood-brain barrier (BBB) in CPTs [12].
Brain edema occurs when plasma-like fluid enters the brain’s extracellular space through impaired capillary endothelial tight junction abnormalities responsible for the increased permeability of tumor endothelial tight junctions [8]. The endothelial tight junction proteins and AQP4 increasing epithelial impermeability strengthen epithelial barrier features and represent targets for the design of novel drugs to treat brain tumor edema [14]. The transmembrane proteins of the Claudin (Cld) family play a crucial role in determining the efficiency and selectivity of TJs and in intercellular junctions of the cerebral microvascular endothelium defined as the differences in a degree of epithelial permeability [8-10]. The subfamily of claudins forming charge- and size-selective pores provides selective paracellular diffusion. Alterations of TJ protein composition have been observed during different processes like stroke, inflammation, infectious disease, and neoplasms [9]
Claudin-5 is the predominant claudin expressed in endothelial tight junctions [8]. Endothelial tight junctions appear to be particularly important in vascular endothelium of the blood-brain barrier [9], aberrant expression of claudins is also common in epithelial cancers. Different cancer sites and histological subtypes seem to affect different claudins. Claudins-1, -2, -11, occludin, and ZO-1 are present in epithelial TJs of the choroid plexus, whereas claudins-1, -5, -11, occludin, and ZO-1 form the TJ of the BBB [9,15,16]. We observed that the expression of claudin 5 has a strong membrane-positive immunoreaction in CPC and negative in the epithelial cells in CPP. This is considered an anomalous expression in CPCs.
E-cadherin is found mainly in the epithelia, where it promotes tight cell-cell associations known as adherens junctions [17]. E-cadherin is a calcium-dependent adhesion glycoprotein; loss of function may increase cellular proliferation, migration, and invasion [17]. Although present on the basolateral surface of most CPPs, it has decreased expression in ACPP and CPCs, implicating loss of function as an important step in malignant progression.
NCPC strongly expresses E-cadherin 5 and N-cadherin 9 as adhesion molecules [17]. N-cadherin is in neural tissue and fibroblasts, where it is thought to mediate a less stable and more dynamic pattern of cell-cell adhesion. The lack of N-cadherin expression has been associated with dissemination and invasion in CPCs. E-cadherin expression tended to be expressed greater in grade I than in grade III CPTs. However, the loss of E-cadherin expression is considered one of the key steps in tumor progression and invasion and is considered among histologic diagnostic criteria [16]. In this paper, we observe a greater expression of E-cadherin and N-cadherin in CPP, than in CPC, and that the expression of N-cadherin was variable, with no prognostic value in TPC. The loss of expression in CPC was also an important factor to note [18,19]. Additionally, aberrant cytoplasmic or nuclear expression of both E-cadherin and β-catenin was often observed in CPTs. The disseminated CPC case was positive for E-cadherin and β-catenin, but negative for N-cadherin [19]. The overexpression of N-cadherin is an acquired change occurring in the Epithelial-to-Mesenchymal Transition (EMT) affecting epithelial tumors [19].
An Extracellular Matrix (ECM) is an acellular three-dimensional macromolecular network composed of collagens, proteoglycans/glycosaminoglycans, elastin, fibronectin, laminin, and several other glycoproteins. Matrix components bind each other as well as cell adhesion receptors forming a complex network in which cells reside in all tissues and organs. Cell surface receptors transduce signals into cells from ECM, which regulate diverse cellular functions, such as survival, growth, and migration, by several matrix-degrading enzymes during normal and pathological conditions. This process involves quantitative and qualitative changes in the ECM [21]. Abnormal ECM dynamics lead to deregulated cell proliferation and invasion, failure of cell death, and loss of cell differentiation, ECM is represented, primarily, by a highly specialized ECM structure, Basement Membrane (BM) [22]. The ECM interacts with cells to regulate diverse functions, including proliferation, migration, and differentiation, it is a dynamic structure that is constantly remodeled to control tissue homeostasis [23].
BM acts as a barrier separating the epithelium from the surrounding stroma and is a dynamic thin sheet-like structure of extracellular matrix that provides a supporting structure on which epithelial and endothelial cells reside [11], further defines the tumor microenvironment and provides significant host-derived regulatory signals during the progression of tumor growth and metastasis [11-14]. The major component of the BM is known to be type IV collagen (Col IV or Col4) is a type of collagen found primarily in the basal lamina. Type IV collagen, along with laminin, plays an important role in cell adhesion, migration, differentiation, and growth. In this regard, degradation of type IV collagen can occur under both physiological and pathological conditions [14]. Type IV collagen degradation products also play an important role during angiogenesis, tissue remodeling, and cancer progression [14], and instruct cell polarity, regulate cell fate decisions, direct cell migrations, and harbor growth factors that mediate a plethora of cellular activities Given the essential roles for BMs in cell and tissue function [24]. Collagenases are enzymes that break the peptide bonds in collagen [15,16], we found a collagen fiber proliferation and cluster formation, with an increase in the expression of anti-collagenase, vimentin, and laminin, which represents an alteration in ECM, which directly influences the development and maintenance of organ shape due to its mechanical properties. Laminin was detected in a sub-epithelial location in all the choroid plexus tumours although basement membrane fragmentation was seen in the choroid plexus carcinomas
Ultra structurally, we observed loss or endothelial cell polarity, a higher expression of collagen IV in PCP and its loss on CPCS, a direct correlation on the MVD, though it was not statistically significant, nonetheless, it was related to the presence of collagenase, laminin, and MM9, as well as a higher mib.1 index and a loss of the expression of e- and n-cadherins, and an anomalous expression of collagens I and V. This anomaly can be observed and correlated with the ultrastructural findings of cell adherent protein ultrastructures and increased as the histological degree increased.
Protective barriers of the brain are dependent on junctional complexes at the interfaces between blood and the CNS, including epithelial cells of the choroid plexuses (blood-cerebrospinal fluid barrier) and endothelial cells of brain capillaries (blood-brain barrier). It is accepted that in the adult plexus, continuous functional tight junctions between adjacent epithelial cells control access of molecules to the CSF and thence to the brain. The molecular configuration and complexity of these junctions in the developing brain in relationship to their “tightness” (i.e. permeability properties) have been controversial. Nonetheless, the expression of anticollagenase might be related to a protection mechanism of the BBB, though this is the first article related to the molecular behavior of CPTs.
The result of Choroid plexus tumors may be exacerbated by the loss of the basement membrane and the overexpression of the extracellular matrix. Increased production of anticollagenase and Matrix Metalloproteinase 9 can modify the flexibility of the basal membrane of the blood-brain barrier and choroid plexus cancers. Tight junction alterations, cell-cell adherence and apical cilia loss, ciliary clusters, membrane loss, and extracellular matrix proliferation are related to the histological grade of choroid plexus tumors.
Author contributions
Conceptualization: MLTS, LCM, CR; Data curation: CSL, AOP; Formal analysis: MLTS. LCM. CSL; Funding acquisition: MLTS, LCM; Investigation: MLTS, LCHM, EGA; Methodology: MLTS. LCM. CSL; Project administration: MLTS. CR; Resources: MLTS. LCM. CSL, AOP; Supervision: MLTS, EGA; Validation: MLTS, EGA, CR, LCHM; Visualization: MLTS, LCHM; Writing—original draft: MLTS, CR, EGA, LCHM; Writing—review & editing: MLTS, AGA.
- Ruggeri L, Alberio N, Alessandrello R, Cinquemani G, Gambadoro C, Lipani R, Maugeri R, Nobile F, Iacopino DG, Urrico G, Battaglia R. Rapid malignant progression of an intraparenchymal choroid plexus papillomas. Surg Neurol Int. 2018 Jul 5;9:131. doi: 10.4103/sni.sni_434_17. PMID: 30105129; PMCID: PMC6044141.
- Spennato P, De Martino L, Russo C, Errico ME, Imperato A, Mazio F, Miccoli G, Quaglietta L, Abate M, Covelli E, Donofrio V, Cinalli G. Tumors of Choroid Plexus and Other Ventricular Tumors. Adv Exp Med Biol. 2023;1405:175-223. doi: 10.1007/978-3-031-23705-8_7. PMID: 37452939.
- Mihaljevic S, Michalicova A, Bhide M, Kovac A. Pathophysiology of the choroid plexus in brain diseases. Gen Physiol Biophys. 2021 Nov;40(6):443-462. doi: 10.4149/gpb_2021032. PMID: 34897020.
- Thomas C, Soschinski P, Zwaig M, Oikonomopoulos S, Okonechnikov K, Pajtler KW, Sill M, Schweizer L, Koch A, Neumann J, Schüller U, Sahm F, Rauschenbach L, Keyvani K, Proescholdt M, Riemenschneider MJ, Segewiß J, Ruckert C, Grauer O, Monoranu CM, Lamszus K, Patrizi A, Kordes U, Siebert R, Kool M, Ragoussis J, Foulkes WD, Paulus W, Rivera B, Hasselblatt M. The genetic landscape of choroid plexus tumors in children and adults. Neuro Oncol. 2021 Apr 12;23(4):650-660. doi: 10.1093/neuonc/noaa267. PMID: 33249490; PMCID: PMC8041331.
- Adib SD, Hempel JM, Kandilaris K, Grimm F, Zamora RE, Tatagiba M. Surgical management of choroid plexus papilloma of the cerebellopontine and cerebellomedullary angle: classification and strategy. Neurosurg Rev. 2021 Dec;44(6):3387-3397. doi: 10.1007/s10143-021-01506-4. Epub 2021 Feb 24. Erratum in: Neurosurg Rev. 2021 Apr 12;: PMID: 33629235; PMCID: PMC8592964.
- Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, Vynios DH, Orian-Rousseau V, Ricard-Blum S, Schmelzer CEH, Duca L, Durbeej M, Afratis NA, Troeberg L, Franchi M, Masola V, Onisto M. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021 Dec;288(24):6850-6912. doi: 10.1111/febs.15776. Epub 2021 Mar 23. PMID: 33605520.
- Wang W, Li S, Wang X, Wang J, Zhang Y. PbO nanoparticles increase the expression of ICAM-1 and VCAM-1 by increasing reactive oxygen species production in choroid plexus. Environ Sci Pollut Res Int. 2023 Mar;30(14):40162-40173. doi: 10.1007/s11356-022-25109-8. Epub 2023 Jan 6. PMID: 36607576.
- Nyimanu D, Behm C, Choudhury S, Yu ASL. The role of claudin-2 in kidney function and dysfunction. Biochem Soc Trans. 2023 Aug 31;51(4):1437-1445. doi: 10.1042/BST20220639. PMID: 37387353.
- Berndt P, Winkler L, Cording J, Breitkreuz-Korff O, Rex A, Dithmer S, Rausch V, Blasig R, Richter M, Sporbert A, Wolburg H, Blasig IE, Haseloff RF. Tight junction proteins at the blood-brain barrier: far more than claudin-5. Cell Mol Life Sci. 2019 May;76(10):1987-2002. doi: 10.1007/s00018-019-03030-7. Epub 2019 Feb 7. PMID: 30734065.
- Raya-Sandino A, Lozada-Soto KM, Rajagopal N, Garcia-Hernandez V, Luissint AC, Brazil JC, Cui G, Koval M, Parkos CA, Nangia S, Nusrat A. Claudin-23 reshapes epithelial tight junction architecture to regulate barrier function. Nat Commun. 2023 Oct 5;14(1):6214. doi: 10.1038/s41467-023-41999-9. PMID: 37798277; PMCID: PMC10556055.
- Arapatzi C, Rouni G, Kostourou V. Vascular cell-matrix adhesion in development and cancer. Int J Dev Biol. 2022;66(1-2-3):103-113. doi: 10.1387/ijdb.210204vk. PMID: 34881799.
- Taddei E, Rosiles A, Hernandez L, Luna R, Rubio C. Apoptosis in the Dentate Nucleus Following Kindling-induced Seizures in Rats. CNS Neurol Disord Drug Targets. 2022;21(6):511-519. doi: 10.2174/1871527320666211201161800. PMID: 34852754.
- Dani N, Herbst RH, McCabe C, Green GS, Kaiser K, Head JP, Cui J, Shipley FB, Jang A, Dionne D, Nguyen L, Rodman C, Riesenfeld SJ, Prochazka J, Prochazkova M, Sedlacek R, Zhang F, Bryja V, Rozenblatt-Rosen O, Habib N, Regev A, Lehtinen MK. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021 May 27;184(11):3056-3074.e21. doi: 10.1016/j.cell.2021.04.003. Epub 2021 Apr 30. PMID: 33932339; PMCID: PMC8214809.
- Moon CS, Moon D, Kang SK. Aquaporins in Cancer Biology. Front Oncol. 2022 Jun 29;12:782829. doi: 10.3389/fonc.2022.782829. PMID: 35847914; PMCID: PMC9278817.
- Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020 Nov 18;17(1):69. doi: 10.1186/s12987-020-00230-3. PMID: 33208141; PMCID: PMC7672931.
- Kakogiannos N, Ferrari L, Giampietro C, Scalise AA, Maderna C, Ravà M, Taddei A, Lampugnani MG, Pisati F, Malinverno M, Martini E, Costa I, Lupia M, Cavallaro U, Beznoussenko GV, Mironov AA, Fernandes B, Rudini N, Dejana E, Giannotta M. JAM-A Acts via C/EBP-α to Promote Claudin-5 Expression and Enhance Endothelial Barrier Function. Circ Res. 2020 Sep 25;127(8):1056-1073. doi: 10.1161/CIRCRESAHA.120.316742. Epub 2020 Jul 15. PMID: 32673519; PMCID: PMC7508279.
- Ohta Y, Fujii M, Takahashi S, Takano A, Nanki K, Matano M, Hanyu H, Saito M, Shimokawa M, Nishikori S, Hatano Y, Ishii R, Sawada K, Machinaga A, Ikeda W, Imamura T, Sato T. Cell-matrix interface regulates dormancy in human colon cancer stem cells. Nature. 2022 Aug;608(7924):784-794. doi: 10.1038/s41586-022-05043-y. Epub 2022 Jul 7. PMID: 35798028.
- Miller AD, Miller CR, Rossmeisl JH. Canine Primary Intracranial Cancer: A Clinicopathologic and Comparative Review of Glioma, Meningioma, and Choroid Plexus Tumors. Front Oncol. 2019 Nov 8;9:1151. doi: 10.3389/fonc.2019.01151. PMID: 31788444; PMCID: PMC6856054.
- Muscatello LV, Avallone G, Serra F, Seuberlich T, Mandara MT, Sisó S, Brunetti B, Oevermann A. Glomeruloid Microvascular Proliferation, Desmoplasia, and High Proliferative Index as Potential Indicators of High Grade Canine Choroid Plexus Tumors. Vet Pathol. 2018 May;55(3):391-401. doi: 10.1177/0300985817754124. Epub 2018 Feb 5. PMID: 29402204.
- Gloushankova NA, Zhitnyak IY, Rubtsova SN. Role of Epithelial-Mesenchymal Transition in Tumor Progression. Biochemistry (Mosc). 2018 Dec;83(12):1469-1476. doi: 10.1134/S0006297918120052. PMID: 30878022.
- LaFoya B, Munroe JA, Miyamoto A, Detweiler MA, Crow JJ, Gazdik T, Albig AR. Beyond the Matrix: The Many Non-ECM Ligands for Integrins. Int J Mol Sci. 2018 Feb 2;19(2):449. doi: 10.3390/ijms19020449. PMID: 29393909; PMCID: PMC5855671.
- Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. 2019 Jun;36(3):171-198. doi: 10.1007/s10585-019-09966-1. Epub 2019 Apr 11. PMID: 30972526.
- Hinneh JA, Gillis JL, Mah CY, Irani S, Shrestha RK, Ryan NK, Atsushi E, Nassar ZD, Lynn DJ, Selth LA, Kato M, Centenera MM, Butler LM. Targeting hyaluronan-mediated motility receptor (HMMR) enhances response to androgen receptor signalling inhibitors in prostate cancer. Br J Cancer. 2023 Oct;129(8):1350-1361. doi: 10.1038/s41416-023-02406-8. Epub 2023 Sep 6. PMID: 37673961; PMCID: PMC10575850.
- Ramos-Lewis W, LaFever KS, Page-McCaw A. A scar-like lesion is apparent in basement membrane after wound repair in vivo. Matrix Biol. 2018 Dec;74:101-120. doi: 10.1016/j.matbio.2018.07.004. Epub 2018 Jul 5. PMID: 29981372; PMCID: PMC6250587.
- Ramos-Lewis W, Page-McCaw A. Basement membrane mechanics shape development: Lessons from the fly. Matrix Biol. 2019 Jan;75-76:72-81. doi: 10.1016/j.matbio.2018.04.004. Epub 2018 Apr 12. PMID: 29656148; PMCID: PMC6185827.
- Wakamatsu K, Chiba Y, Murakami R, Matsumoto K, Miyai Y, Kawauchi M, Yanase K, Uemura N, Ueno M. Immunohistochemical expression of osteopontin and collagens in choroid plexus of human brains. Neuropathology. 2022 Apr;42(2):117-125. doi: 10.1111/neup.12791. Epub 2021 Dec 28. PMID: 34964160; PMCID: PMC9546339.
- Maeba T, Yonezawa T, Ono M, Tomono Y, Heljasvaara R, Pihlajaniemi T, Inagawa K, Oohashi T. Collagen XVIII Deposition in the Basement Membrane Zone beneath the Newly Forming Epidermis during Wound Healing in Mice. Acta Med Okayama. 2019 Apr;73(2):135-146. doi: 10.18926/AMO/56649. PMID: 31015748.