More Information
Submitted: September 01, 2022 | Approved: September 15, 2022 | Published: September 16, 2022
How to cite this article: Keshavarz S, Emamzadeh E, Sardari D, Darki SY, Kabirian M. Boron neutron capture therapy for the treatment of lung cancer and assessment of dose received by organs at risk. Arch Pathol Clin Res. 2022; 6: 027-031.
DOI: 10.29328/journal.apcr.1001032
Copyright License: © 2022 Keshavarz S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Lung cancer; BNCT; Total biological dose; Boron concentration; Organ at risk
Boron neutron capture therapy for the treatment of lung cancer and assessment of dose received by organs at risk
Sajad Keshavarz1, Elnaz Emamzadeh1, Dariush Sardari1*, Sepideh Yazdani Darki2 and Marzieh Kabirian1
1Department of Medical Radiation Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Department of Physics, University of Yazd, Yazd, Iran
*Address for Correspondence: Dariush Sardari, Department of Medical Radiation Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran, Email: [email protected]; [email protected]
Recent studies on boron neutron capture therapy (BNCT) have focused on investigating the appropriate neutron sources based on accelerators for neutron production, such as 7Li(p,n)7 Be. The therapeutic abilities of BNCT have been studied for the possible treatment of lung cancer using thermal and epithermal neutron beams. For neutron transport, the Monte Carlo N-particle transport code was used, and doses in the organs of different Oak Ridge National Laboratory phantoms were evaluated. The right lung was meshed with voxels to obtain depth-dose distributions using 1 eV, 10 eV, 100 eV, 1 keV, 5 keV, 8 keV and 10 keV energy sources. These results suggest that BNCT with an epithermal neutron beam can be used to treat lung cancer. By evaluating the biological dose rate and dose-depth distribution curves in healthy tissues and tumors by simulating a lung phantom, the quantities in the phantom were also evaluated. Our calculations show that with increasing boron concentration applied to the tumor, the dose is increased and the 100 eV energy source has the greatest effect on the tumor dose.
The incidence of lung cancer is increasing worldwide and it has been the leading cause of cancer-related mortality. The treatment methods for lung cancer include surgery, chemotherapy and radiotherapy [1].
Boron neutron capture therapy (BNCT) is a type of radiation therapy that uses isotope 10B, as well as neutron beams. BNCT is one of the most important methods of treating some cancers, including the brain [2,3], liver [4] and other tumors [5]. In recent years, researchers have proposed the use of this method to treat lung cancer because of its superiority to conventional radiotherapies [1]. The purpose of radiotherapy techniques is to reduce the absorbed dose in healthy tissues [6]. The basis of BNCT is:-1) neutron bombardment of the intended area at the appropriate intensity and energy and 2) 10B compound fully concentrates in tumor cells [7]. In this treatment, boron first concentrates inside the tumor as a special compound labeled by tumor seeker or biomarker materials. A beam of epithermal neutrons with appropriate energy and intensity then irradiates the tumor area. The energy of epithermal neutrons decreases as they pass through different tissues and are converted into thermal neutrons. As a result of the reaction of thermal neutrons with boron in the tumor, alpha and lithium particles are produced [8,9]. These have high energy and low range (approximately the size of a cell) and release their energy within the tumor which kills cancer cells [2,10].
In the normal state, the lung contains a large amount of air, which does not attenuate neutrons. Consequently, the epithermal neutrons used in BNCT can move into the thorax to treat tumors at greater depths. In 2006, Suzuki, et al. first reported the use of BNCT in thoracic cancer and conducted a dosimetric study to evaluate the feasibility of BNCT for malignant pleural mesothelioma [5,11].
Krstic, et al. [12] calculated the dose distribution in BNCT for liver cancer and reported that in BNCT, using an epithermal neutron beam could be applied for liver cancer treatment. In another study by this research group, the lung dose in a male ORNAL phantom provided by BNCT was calculated, and the obtained results indicated that lung cancer could be treated using BNCT [13]. Moghaddasi, et al. [14] compared clinical target volume (CTV) and BNCT-based methods for the treatment of brain tumors, in which BNCT was found to lead to more efficient cell destruction.
Superficial tumors require thermal neutrons, while epithermal neutron energy spectra, i.e., in the 1 eV-10 keV, are suitable for deep tumors. In this case, neutrons complete their slowing-down process through the healthy tissue around the tumor, causing minor biological damage, and in the proximity of the malignant tissue to the range of thermal energies, their interaction with 10B increases the damage to the tumor [9].
Therefore, the determination of boron concentration is an essential requirement for the treatment of BNCT and is achieved through the appropriate calculation of doses absorbed by other organs of the body.
Trivillin, et al. (2019) evaluated the therapeutic efficacy and toxicity of BNCT in an experimental model of lung metastasis of colon carcinoma in BDIX rats. Their results showed no toxicity and lung metastases, as revealed using BNCT [10]. Bykov, et al. (2021) at the Budker Institute of Nuclear Physics (BINP) designed a new detector and measured the dose-depth profile in a water phantom [15].
This paper provides the results of Monte Carlo simulations of depth-dose distributions for the possible treatment of lung cancer using BNCT. Whole-body calculations were also performed on doses to other organs when simulating patients with lung tumors.
In the present study, the computational model of the Oak Ridge National Laboratory (ORNL) phantom [16] was used to calculate the number of body organs to simulate tumors in the lungs. Simulation of neutron transport from the source to the target organs was used to calculate the designed thermal, epithermal and fast neutron fluxes, fast neutron doses, and gamma-ray dose at the beam exit and in the phantom lung.
MCNPX simulation cards, such as cell, surface and material descriptions; the position of each tool; and definitions and features of sources, were defined in the input file according to their properties [17].
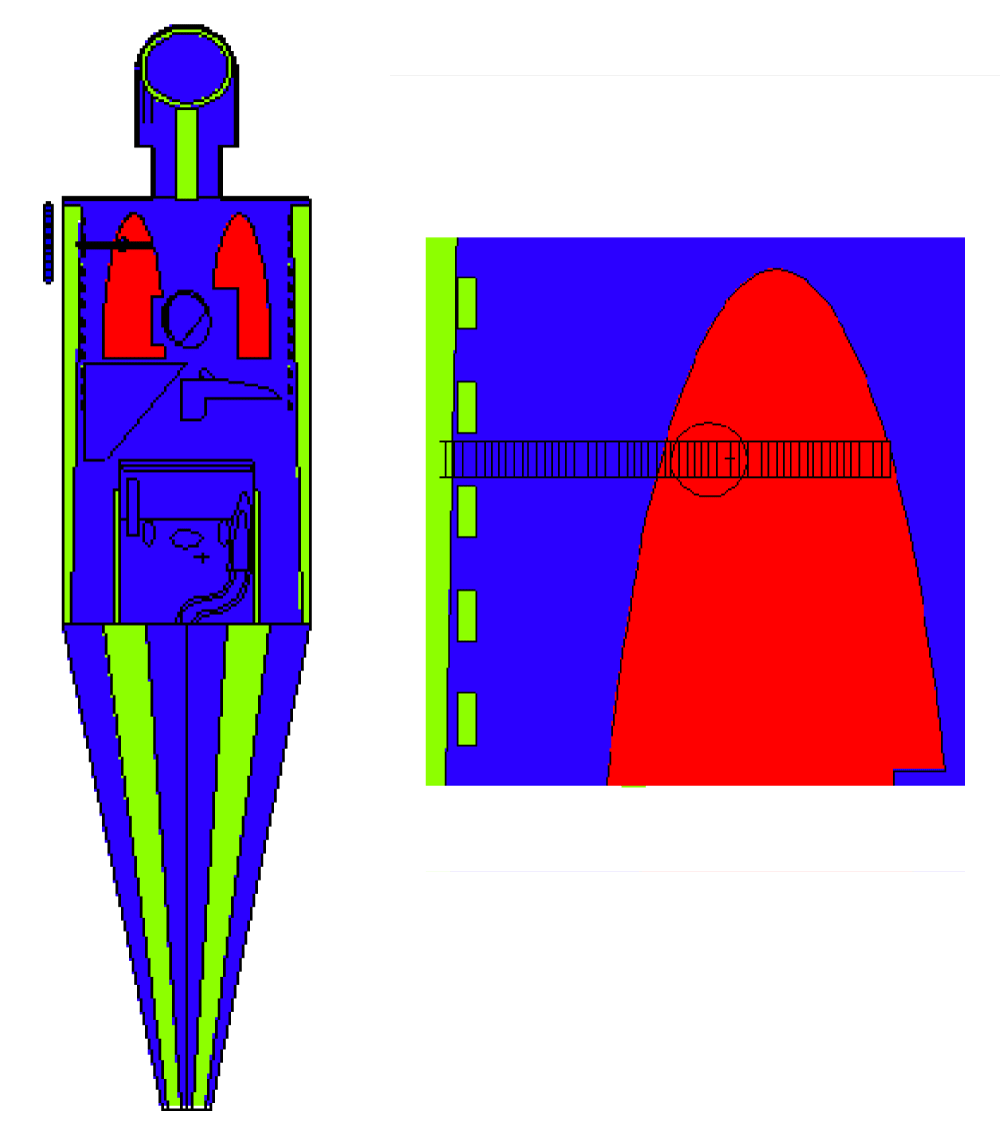
Geometry simulation was carried out utilizing a sphere with a 1-cm radius as a tumor in the upper lobe of the right lung. The right lung was segmented with a 0.5-cm radius and 0.2 cm thickness. The geometry and phantom are shown in Figure 1.
Figure 1: A schematic cross-sectional view of the lung phantom and ORNL MIRD human phantom with a typical tumor located in the upper lobe.
A circular neutron source with a 6-cm radius irradiated the phantom from the right side with energies of 1 eV, 10 eV, 100 eV, 1 keV, 5 keV, 8 keV and 10 keV and 1010s-1 of intensity as the representation of thermal neutrons, epithermal neutrons and fast neutrons. In the present study, the boron concentrations in cancerous and normal lung tissues were 65 and 18 ppm, respectively. To investigate the effect of boron concentration, other cases were considered for 55 and 15 ppm, 45 and 12 ppm, 30 and 8 ppm and, 25 and 6 ppm of boron for tumoral and normal tissues respectively.
The calculation of the total absorbed dose (Htotal) in tumoral and healthy tissues following BNCT was composed of four radiation dose components. These were the gamma-ray dose (Dγ), arising from contamination of the neutron beam and dose from photons induced by neutron capture reactions in tissues; fast neutron dose (Dn_fast), which is due to the proton recoil generated as a result of 1H(n,n) 1H reactions; thermal neutron dose (Dn_thermal), which is a dose produced by thermal neutron capture in the 14N(n,p)14 C reaction; and boron dose (DB), which is due to the interaction of neutrons with boron when the neutron beam impinges on boron-bearing lung tissue.
To consider the relative biological effects, the four physical dose components should be multiplied by an appropriate weighting factor, as presented in Table 1 [18,19] and ICRP60 [20].
| Table 1: RBE and CBE factors used in the conversion of physical dose to photon-equivalent dose. | ||
| BNCT dose component | Tumor | Lung |
| 10B (n,α) 7Li | 3.8 | 1.5 |
| 14N (n,p)1 ] 14C | 3.0 | 2.2 |
| Fast neutron | 3.0 | 2.2 |
| Photons | 1.0 | 1.0 |
The material compositions of the lung and kerma coefficients were defined based on the ICRU Report 46 [21].
In the present work, we provide the results of computational dosimetry for several different neutron source energies (1, 10, and 100 eV and 1, 5, 8, and 10 keV). In addition, various concentrations of 10B were tested. When the tumor was located in the left lung, the total dose in the right lung left lung, heart, stomach, bladder, liver, thyroid, skin, brain, small intestine, kidney, pancreas, spleen, thymus, uterus, right breast, left breast and gall bladder was calculated.
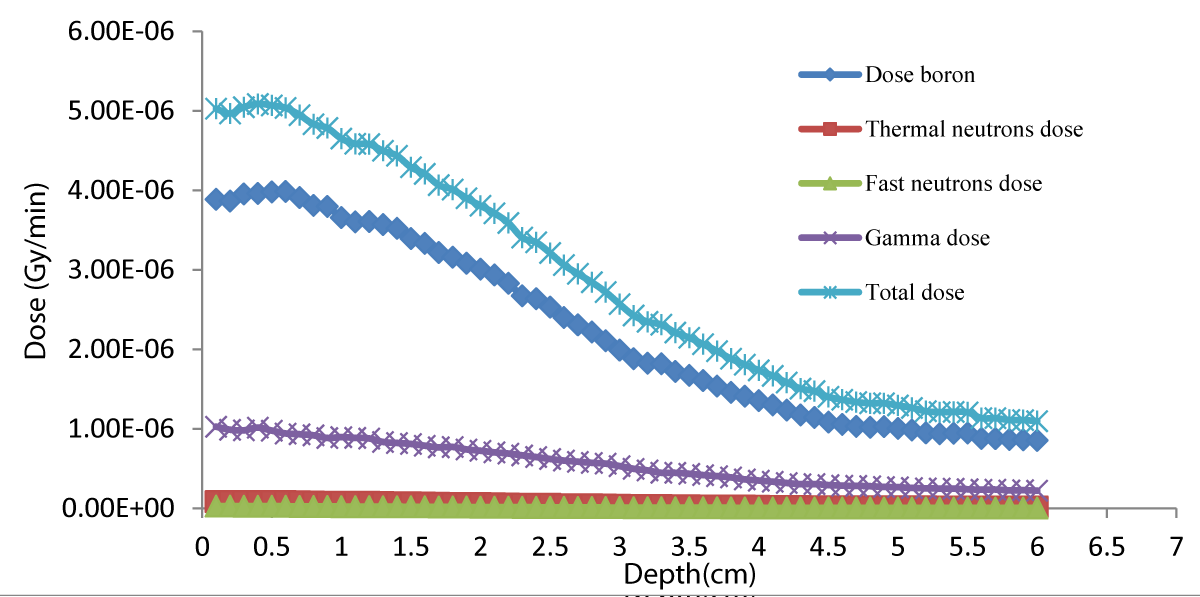
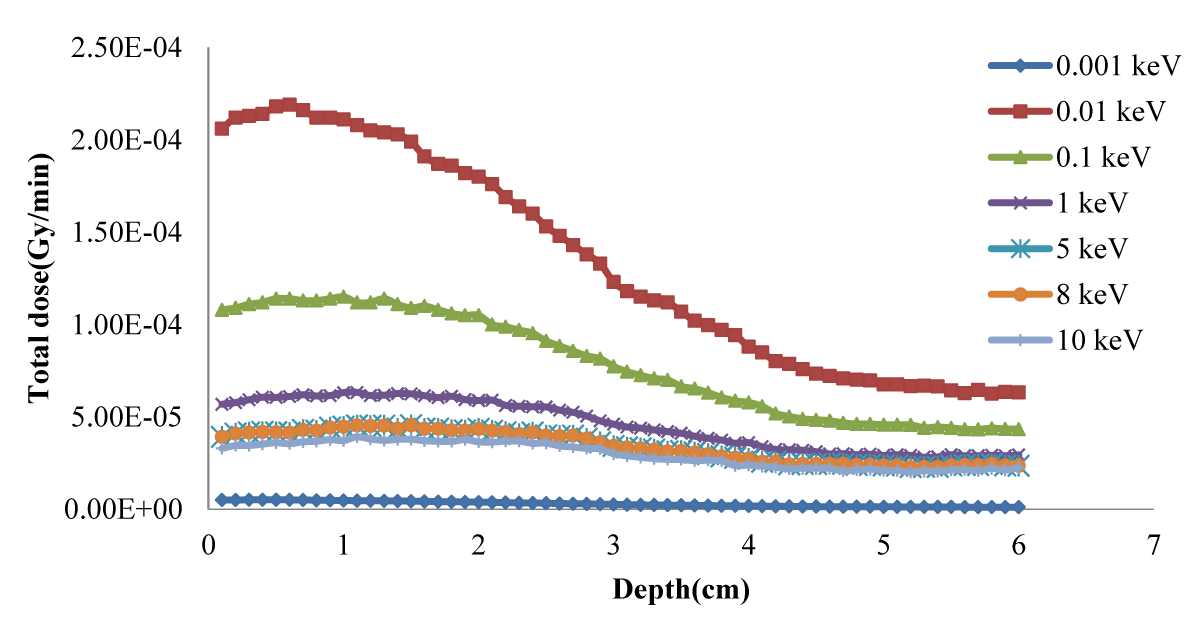
Figure 2 shows the total dose curves in normal tissue from the radiation of the simulated neutron beam to the lung phantom. Figure 3 shows the total depth-dose curve for different neutron source energies and shows that 100 eV has the highest dose rate.
Figure 2: Depth dose distributions in healthy tissue with 1eV neutron energy.
Figure 3: Total dose vs. depth for different neutron source energies.
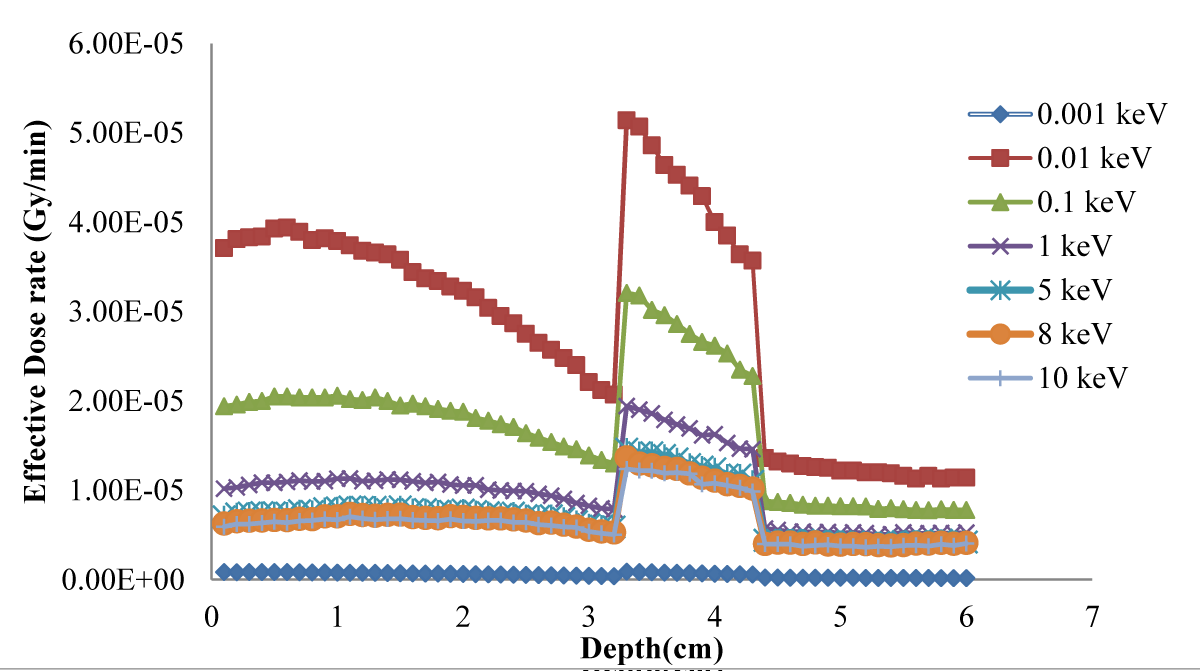
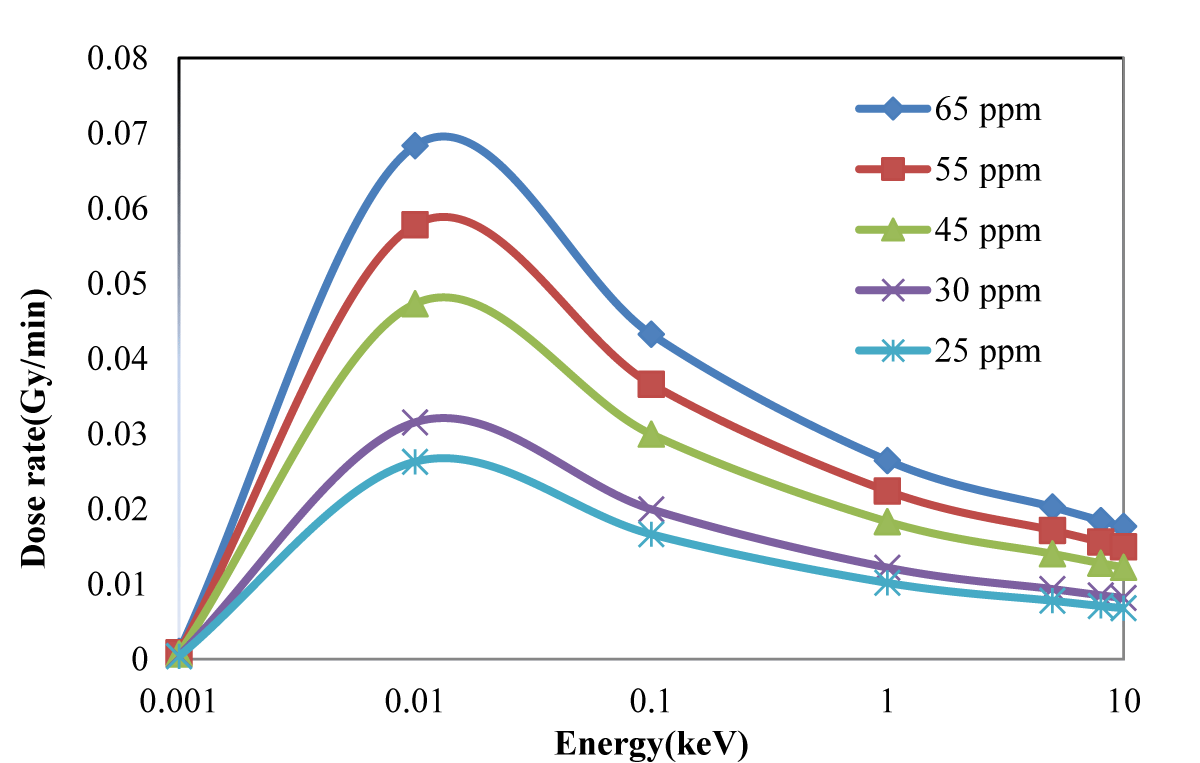
The effective dose delivered to the tumor and lung was evaluated for a simulated phantom of the human body containing the lung for different neutron source energy and 65 ppm and 18 ppm of 10B as shown in Figure 4. The evaluated energy-dose profiles for tumors with 25, 30, 45, 55 and 65 ppm of concentration are shown in Figure 5.
Figure 4: Effective dose delivered to the tumor and lung for a simulated phantom of the human body containing the lung for different neutron source energies
Figure 5: Dose rate for different concentrations of 10B in the tumor.
The tumor tissue presented in Figure 1 was irradiated with three separate beams of thermal, fast and epithermal neutrons.
Figure 1 shows that the dose rapidly decreases with increasing depth; in the tumor in Figure 3, the dose is higher because of higher concentrations of 10B. Regarding the concentration of 10B in the tumor, 65 ppm provided the highest dose. Notably providing an epithermal neutron beam was more convenient. Neutrons have sufficient energy to deliver far greater doses in the tumor tissue than in the healthy tissue. Figures 3-5 shows that 100 eV of source energy has the greatest effect on tumors and killing cancerous cells.
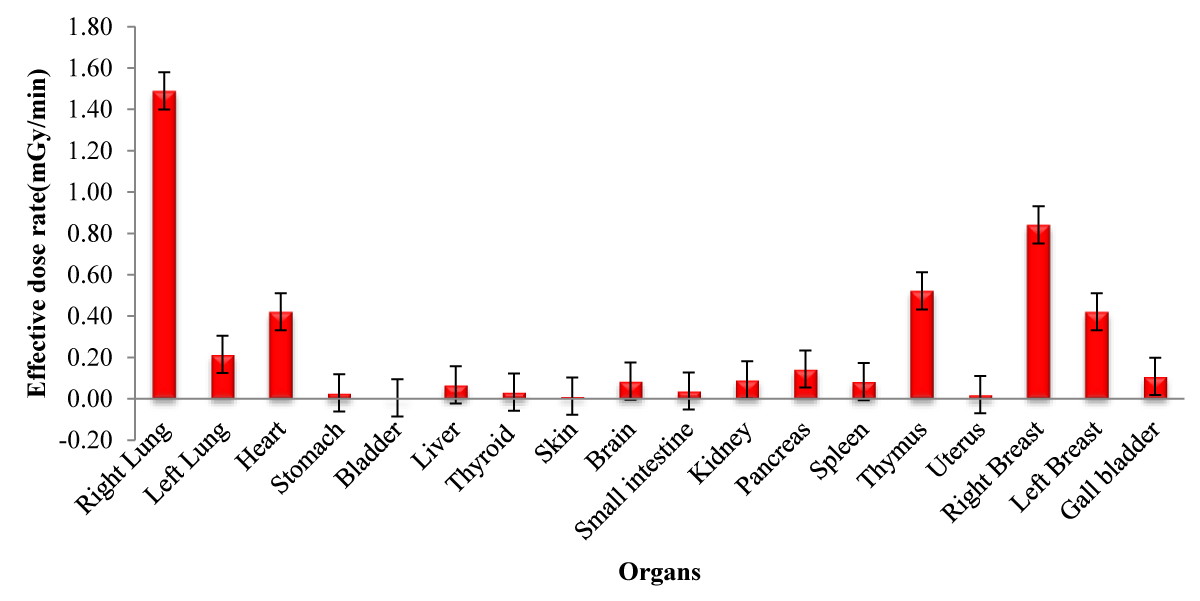
In the present study, the right and left lungs, heart, skin, left and right breast, liver, kidney, brain, stomach, gallbladder, thymus, small intestine, thyroid, pancreas, uterus, bladder and spleen were also in the vicinity of the beam. The effective doses in these organs for 65 ppm 10B at different energies and 100 eV are shown in Table 2 and Figure 6 respectively.
| Table 2: Effective dose (mGy) in tissues of different energy (keV) at concentration of 65 ppm, and 18 ppm in the tumor and healthy tissue, respectively. | |||||||
| Neutron Energy (keV) Organs | 0.001 | 0.01 | 0.1 | 1 | 5 | 8 | 10 |
| Right Lung | 1.806 | 1.497 | 1.489 | 1.486 | 1.142 | 1.083 | 1.058 |
| Left Lung | 0.220 | 0.217 | 0.214 | 0.214 | 0.189 | 0.185 | 0.183 |
| Heart | 0.419 | 0.420 | 0.421 | 0.423 | 0.376 | 0.365 | 0.361 |
| Stomach | 0.030 | 0.029 | 0.028 | 0.028 | 0.025 | 0.024 | 0.024 |
| Bladder | 0.005 | 0.004 | 0.004 | 0.004 | 0.003 | 0.003 | 0.003 |
| Liver | 0.069 | 0.068 | 0.067 | 0.067 | 0.059 | 0.057 | 0.057 |
| Thyroid | 0.033 | 0.033 | 0.032 | 0.032 | 0.027 | 0.026 | 0.026 |
| Skin | 0.013 | 0.013 | 0.013 | 0.012 | 0.009 | 0.009 | 0.009 |
| Brain | 0.092 | 0.089 | 0.085 | 0.085 | 0.068 | 0.065 | 0.063 |
| Small intestine | 0.043 | 0.040 | 0.038 | 0.037 | 0.032 | 0.031 | 0.030 |
| Kidney | 0.098 | 0.094 | 0.091 | 0.090 | 0.079 | 0.077 | 0.076 |
| Pancreas | 0.153 | 0.148 | 0.144 | 0.143 | 0.126 | 0.126 | 0.124 |
| Spleen | 0.089 | 0.085 | 0.083 | 0.080 | 0.070 | 0.069 | 0.069 |
| Thymus | 0.522 | 0.529 | 0.522 | 0.520 | 0.462 | 0.442 | 0.437 |
| Uterus | 0.024 | 0.022 | 0.020 | 0.019 | 0.016 | 0.016 | 0.016 |
| Right Breast | 0.857 | 0.849 | 0.841 | 0.837 | 0.680 | 0.648 | 0.635 |
| Left Breast | 0.428 | 0.425 | 0.421 | 0.419 | 0.340 | 0.324 | 0.318 |
| Gall bladder | 0.118 | 0.111 | 0.108 | 0.106 | 0.094 | 0.092 | 0.090 |
Figure 6: Effective dose rate for different organs with 100 eV neutron source energy and 65 ppm 10B concentration..
The results obtained in this study were in good agreement with those reported by Kabirian, et al. [22,23].
BNCT is developing into an excellent lung cancer treatment. Here we showed that 10 eV epithermal neutrons are a better candidate for this purpose. Because the computed dose is per source neutron, higher exposure to epithermal neutrons for the same flux of the thermal neutrons can give rise to the same dose in tumor tissue, whereas the dose in healthy tissue will be far lower with epithermal neutrons. The surrounding organs in this study received lower doses than the target tissue while the beam of neutrons was collimated. An increased 10B concentration and optimum range of neutron source energy can elevate the tumor dose and improve BNCT treatment. Therefore, this is a very powerful method because of the selective uptake of boron.
Declaration
This work was carried out using computational software and no human or animal samples or species were utilized.
The authors declare adherence to the Declaration of Helsinki in the present work.
We declare no conflict of interest among authors and between authors and relevant institutions.
All co-authors are aware of the content of the manuscript and its submission.
- Wu S, Geng C, Tang X, Bortolussi S, Han Y, Shu D, Gong C, Zhang X, Tian F. Dosimetric impact of respiratory motion during boron neutron capture therapy for lung cancer. Radiation Physics and Chemistry. 2020;168, 108527. https://doi.org/10.1016/j.radphyschem.2019.108527
- Taheri A, Sardari D, Sayyareh R, Sadeghi M. Dose mapping simulation and BSA design for improving the dosimetry accuracy for research reactor BNCT. Journal of Neutron Research. 2019; 1-11. 10.3233/JNR-180082
- Pozzi EC, Cardoso JE, Colombo LL, Thorp S, Monti Hughes A, Molinari AJ, Garabalino MA, Heber EM, Miller M, Itoiz ME, Aromando RF, Nigg DW, Quintana J, Trivillin VA, Schwint AE. Boron neutron capture therapy (BNCT) for liver metastasis: therapeutic efficacy in an experimental model. Radiat Environ Biophys. 2012 Aug;51(3):331-9. doi: 10.1007/s00411-012-0419-8. Epub 2012 Apr 28. PMID: 22544068.
- Ichikawa G, Tsuchida K, Kiyanagi Y, Ishikawa A, Hirata Y, Yoshihashi S, Watanabe K, Uritani A, Hamano T, Ogawara R, Igawa K, Suda M. Development of thermal neutron moderator for testing boron agents for Boron Neutron Capture Therapy (BNCT). Journal of Instrumentation. 2019; 14:06;T06010. 10.1088/1748-0221/14/06/T06010.
- Suzuki M. Boron neutron capture therapy (BNCT): a unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int J Clin Oncol. 2020 Jan;25(1):43-50. doi: 10.1007/s10147-019-01480-4. Epub 2019 Jun 5. PMID: 31168726.
- Keshavarz S, Sardari D. Different distributions of gold nanoparticles on the tumor and calculation of dose enhancement factor by Monte Carlo simulation. Nuclear Energy and Technology. 2019; 5:361. 10.3897/nucet.5.39096.
- Kaur M, Singh P, Singh K, Gaharwar US, Meena R, Kumar M, Nakagawa F, Wu Sh, Suzuki M, Nakamura H, Kumar A. Boron nitride (10BN) a prospective material for treatment of cancer by boron neutron capture therapy (BNCT). Materials Letters. 2020;259: 126832. https://doi.org/10.1016/j.matlet.2019.126832.
- Karaoglu A, Arce P, Obradors D, Lagares JI, Unak P. Calculation by GAMOS/Geant4 simulation of cellular energy distributions from alpha and lithium-7 particles created by BNCT. Appl Radiat Isot. 2018 Feb;132:206-211. doi: 10.1016/j.apradiso.2017.11.021. Epub 2017 Nov 20. PMID: 29183761.
- Shaaban I, Albarhoum M. Design calculation of an epithermal neutronic beam for BNCT at the Syrian MNSR using the MCNP4C code. Progress in Nuclear energy. 2015;78: 297-302. https://doi.org/10.1016/j.pnucene.2014.10.005
- Trivillin VA, Serrano A, Garabalino MA, Colombo LL, Pozzi EC, Hughes AM, Curotto PM, Thorp SI, Farías RO, González SJ, Bortolussi S, Altieri S, Itoiz ME, Aromando RF, Nigg DW, Schwint AE. Translational boron neutron capture therapy (BNCT) studies for the treatment of tumors in lung. Int J Radiat Biol. 2019 May;95(5):646-654. doi: 10.1080/09553002.2019.1564080. Epub 2019 Feb 22. PMID: 30601686.
- Quah SC. Boron neutron capture therapy in the treatment of lung cancer. Journal of Xiangya Medicine. 2018; 3: 29. 10.21037/jxym.2018.06.02.
- Krstic D, Jovanovic Z, Markovic V, Nikezic D, Urosevic V. MCNP simulation of the dose distribution in liver cancer treatment for BNC therapy. Open Physics. 2014;12(10): 714–718. https://doi.org/10.2478/s11534-014-0507-2.
- Krstic D, Markovic VM, Jovanovic Z, Milenkovic B, Nikezic D, Atanackovic J. Monte Carlo calculations of lung dose in ORNL phantom for boron neutron capture therapy. Radiat Prot Dosimetry. 2014 Oct;161(1-4):269-73. doi: 10.1093/rpd/nct365. Epub 2014 Jan 16. PMID: 24435912.
- Moghaddasi L, Bezak E. Development of an integrated Monte Carlo model for glioblastoma multiforme treated with boron neutron capture therapy. Sci Rep. 2017 Aug 1;7(1):7069. doi: 10.1038/s41598-017-07302-9. PMID: 28765533; PMCID: PMC5539248.
- Bykov TA, Kasatov DA, Koshkarev AM, Makarov AN, Leonov VV, Porosev VV, Verkhovod GD. Evaluation of depth-dose profiles in a water phantom at the BNCT facility at BINP. Journal of Instrumentation. 2021; 16(10): P10016.
- Large MJ, Malaroda A, Petasecca M, Rosenfeld AB, Guatelli S. Modelling ICRP110 Adult Reference Voxel Phantoms for dosimetric applications: Development of a new Geant4 Advanced Example. In Journal of Physics: Conference Series IOP Publishing. 2020;1661(1): 012021. 10.1088/1742-6596/1662/1/012021.
- Darki SY, Keshavarz S. Studies on mass attenuation coefficients for some body tissues with different medical sources and their validation using Monte Carlo codes. Nuclear Science and Techniques. 2020; 31(12):1-15. https://doi.org/10.1007/s41365-020-00827-1.
- Coderre JA, Morris GM. The radiation biology of boron neutron capture therapy. Radiat Res. 1999 Jan;151(1):1-18. PMID: 9973079.
- Kiger JL, Kiger WS, Patel H, Binns PJ, Riley KJ, Hopewell JW, Harling OK, Coderre JA. Effects of boron neutron capture irradiation on the normal lung of rats. Appl Radiat Isot. 2004 Nov;61(5):969-73. doi: 10.1016/j.apradiso.2004.05.021. PMID: 15308177.
- 1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP. 1991;21(1-3):1-201. PMID: 2053748.
- White DR, Griffith RV, Wilson IJ. Report 46. Journal of the International Commission on Radiation Units and Measurements. 1992;1: NP-NP. https://doi.org/10.1093/jicru/os24.1.Report46.
- Kabirian M, Sardari D, Babapour F. Determination of proper neutron beam energy spectrum for penetration depth in liver BNCT. International Academic Journal of Science and Engineering. 2016; 3:106-114.
- Zabihzadeh M, Rahimli F, Behrooz MA, Danyaei A, Shabazian H. Evaluation of Dose distribution in lung tumor radiotherapy with boron neutron capture therapy. Iranian Journal of Medical Physics. 2021; 18: 63-69.