More Information
Submitted: December 15, 2021 | Approved: January 03, 2022 | Published: January 04, 2022
How to cite this article: Lombard CM. The identification of the true nature of pseudofungus structures as polyurethane catheter fragments. Arch Pathol Clin Res. 2022; 6: 005-008.
DOI: 10.29328/journal.apcr.1001029
Copyright License: © 2022 Lombard CM. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pseudofungus; Pseudofungi; Polyurethane catheter fragments; Pathogenesis; Pathology
The identification of the true nature of pseudofungus structures as polyurethane catheter fragments
Charles M Lombard*
Department of Pathology, El Camino Hospital Mountain View, CA 94040, USA
*Address for Correspondence: Charles M Lombard, MD, Department of Pathology GC # 431, 2500 Grant Road, El Camino Hospital Mountain View, CA 94040, USA, Email: [email protected]
Pseudofungus structures in lymph node tissues have been reported on multiple occasions. Despite a variety of investigative tests including histochemical special stains and energy dispersive spectral analysis, the underlying nature and origin of these pseudofungus structures has never been clearly defined. The most common hypothesis suggests that they represent collagen fibers that become coated with iron and calcium. Herein, evidence is given that the pseudofungus structures identified in the lymph node tissues represent fragments of polyurethane catheters. The evidence includes both a comparison of these pseudofungus structures to fragments of polyurethane well documented in the literature and a comparison of polyurethane catheter scrapings to the pseudofungus structures identified in the literature. In both of these comparisons, the morphology of the polyurethane fragments are identical to the pseudofungus structures. This is the first definitive report identifying polyurethane catheter fragments as representing the true nature and etiology of pseudofungus structures in lymph node tissues.
Pseudofungus structures were first described in 1991 [1] and subsequently were reported on multiple occasions [2-9]. Despite a variety of investigative tests including histochemical special stains and energy dispersive spectral analysis, the underlying nature and origin of these pseudofungus structures has never been clearly defined. The most common hypothesis suggests that they represent collagen fibers that become coated with iron and calcium. Herein, the underlying nature of these pseudofungus structures is, for the first time, positively and clearly identified as fragments of polyurethane catheters. A review of the previously reported cases of pseudofungus structures suggests that, although clinical information was limited, and no specific mention of catheter usage was reported, it nevertheless appears probable that there was catheter usage in these patients prior to their surgical procedures where pseudofungus structures were identified.
A case with a large breast mass and enlarged axillary lymph nodes was encountered. A needle biopsy of the breast was performed and highly necrotic tissues were encountered. There was excessive bleeding and an exodus array (Angiodynamics) 8 French multipurpose drainage catheter was inserted. 10 days after the catheter was inserted, a needle biopsy of an axillary lymph node was performed. This showed no evidence of metastatic carcinoma but did show pseudofungus structures in the subcapsular sinus. Because of continued concern for lymph node metastasis, an excisional left axillary sentinel lymph node biopsy was performed 18 days after the initial needle biopsy of the breast and catheter placement. This lymph node again showed no evidence of metastatic carcinoma but did show pseudofungus structures in the lymph node sinuses. 5 months after the initial biopsy and catheter placement, a modified radical mastectomy was performed. Persistent pseudofungus structures were identified in one of the axillary lymph nodes. Routine histologic studies were performed, followed by a critical review of the literature, and direct smear preparations on the multipurpose drainage catheter.
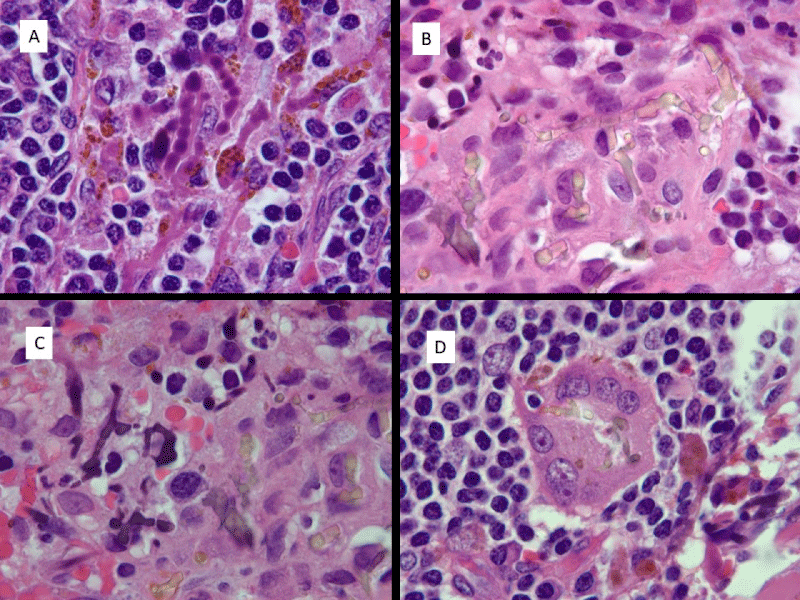
The pseudofungus structures were examined on routine hematoxylin and eosin stains as well as PAS (periodic acid-Schiff), Prussian blue iron stains, von Kossa stains and GMS (Grocott’s methenamine silver) stains. The structures appear similar in all 3 specimens and will be described together. On hematoxylin and eosin stains a dual appearance was noted. The most prevalent appearance was of a basophilic staining hyphal-like structure of similar size to Aspergillus organisms. The branching appears more haphazard than the branching seen in Aspergillus organisms. The pseudofungus structures appear septate. Occasional forms have a beaded appearance. A second appearance is that of a yellowish-brown hyphal-like structure of identical size to the previously described structures, and also showing septation. These structures have a refractile quality, unlike the basophilic structures, although neither of these 2 forms are birefringent. Multinucleate giant cells were also noted and both types of pseudofungus structures were identified within a subset of these giant cells. These histologic features are demonstrated in Figure 1A-D.
Figure 1: Histologic appearance of pseudofungus forms. A) Basophilic, non-refractile beaded and pseudohyphal forms. B) Refractile yellowish-brown pseudohyphal forms. C) Basophilic non-refractile pseudohyphal forms on right and refractile yellowish-brown foms on left. D) Refractile pseudohyphal forms in multinucleate giant cell. (All images are hematoxylin and eosin; 1000 x magnification).
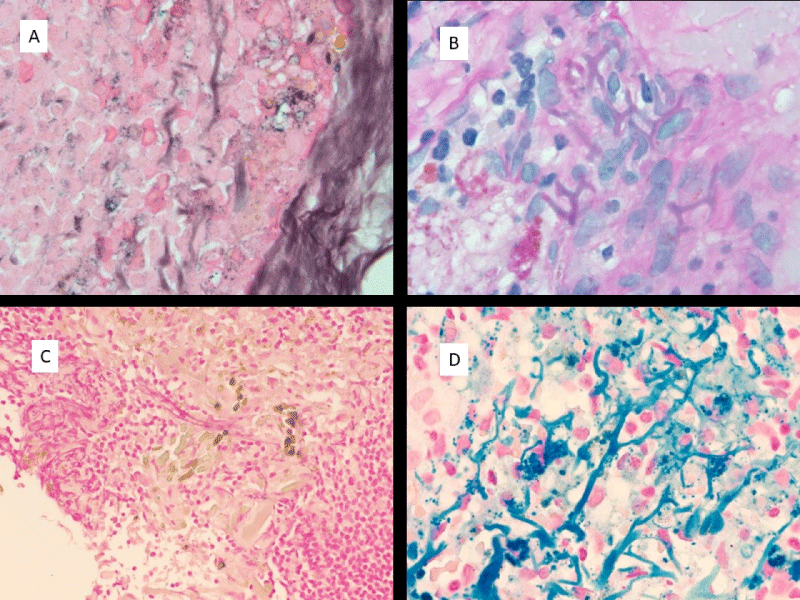
The pseudofungus structures were examined with special histochemical stains. The structures stain strongly with Prussian blue iron stain. They also stain with PAS stain and the positive staining is only weakly preserved following diastase digestion. Von Kossa stain shows positive staining for calcium. GMS stain shows variable staining of these structures. These staining characteristics are tabulated in Table 1 and demonstrated in Figure 2A-D.
| Table 1: Documentation of staining characteristics of pseudofungal structures in lymph node specimens, including both refractile and non-refractile forms. | ||||
| Staining Properites of Pseudofungal Forms | ||||
| Special Stains | PAS | GMS | IRON | VON KOSSA |
| Refractile forms | Weak + | Weak + | + | Negative |
| Non-refractile forms | + | Weak + | + | Negative |
Figure 2: Histochemical staining properties of pseudofungus structures. A) Weak positive GMS staining; B) Weak positive PAS staining; C) Absence of staining with Von Kossa; D) Strong positive staining with Prussian Blue Iron stain. (All images are at 1000 x magnification).
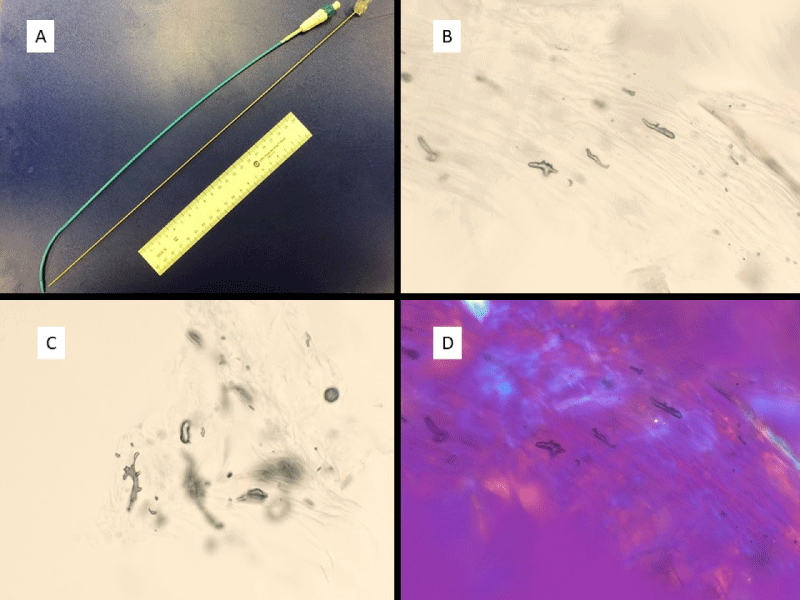
After the first lymph node biopsy in which pseudofungus structures were identified, particular attention was paid to the catheter that was placed for drainage of the hemorrhagic tumor, as it represented the only unusual feature in this patient’s clinical course. The polyurethane nature of the catheter was noted and a review of the literature on polyurethane fragments led to the discovery that these fragments appear identical to the pseudofungus structures [10]. A scrape preparation of the catheter was examined and this demonstrated pseudofungus structures that are identical to the pseudofungus structures identified in the lymph node tissues. The catheter and scrape preparation findings are demonstrated in Figure 3A-D.
Figure 3: Polyurethane catheter and direct smear preparations of catheter scrapings. A) Polyurethane catheter; B,C) Images of catheter fragments from direct catheter scrape preparation; D) Examination of catheter fragments show no significant birefringence. (All microscopic images at 1000 x; Direct smear preparations without any staining).
Since the first description in 1991 [1], the nature of these pseudofungus structures has remained a mystery. The most common theory is that they represent collagen fibers that are coated with iron and calcium. However, the distinctive branching pattern of these structures and their close resemblance to septate hyphal fungal organisms would be highly unusual for collagen fibers. In addition, collagen fibers are very common and identification of these pseudofungus structures is rare. The distinctive appearance of these structures in the first lymph node biopsy specimen led to a close examination of the clinical history for clues as to what might have given rise to these structures. The only unusual feature in the clinical course was the placement of a drainage catheter in the breast for excessive post biopsy bleeding. Recognizing the polyurethane nature of the drainage catheter led to an investigation into the structure of polyurethane fragments and recognition that these fragments appear identical to well described and characterized polyurethane fragments [10]. Confirmation of this finding was completed by examination of a scrape preparation of the drainage catheter which demonstrated pseudofungus structures identical to the structures identified in the lymph node specimens. It is postulated that during placement of the catheter that the metal trocar scraped off small fragments of polyurethane from the internal lining of the catheter and deposited them in the tissue. These fragments subsequently drained to the regional lymph nodes where they were identified on a subsequent biopsy/excision. The sequential lymph node biopsies demonstrate that these pseudofungus fragments reach the draining lymph nodes no later than day 10 after placement of the catheter and persist in the draining lymph nodes for at least 5 months.
Previous descriptions of pseudofungus structures were reviewed with particular attention to the clinical settings. Table 2 displays previous cases where pseudofungus structures were identified. The clinical information regarding the pre-surgical procedures is quite limited and none of these cases has the specific mention of use of a pre-surgical catheter. In all of these cases, it is possible, if not probable that a pre-surgical catheter was used. In one case [8] the clinical scenario is similar to the case reported here. A hemorrhagic breast carcinoma was biopsied with a subsequent resection in which psuedofungal structures were identified in a regional lymph node.
| Table 2: Cases of reported pseudofungal structures including primary tumors, sites of lymph nodes where pseudofungal structures were found and an estimation of the likelihood of pre-surgical catheter use. (LN = lymph node; GIST = Gastrointestinal stromal tumor). | |||
| Case # | 10 tumor/ site | Pseudofungus Site | Likelihood of Catheter usage |
| 11 | Melanoma/shoulder | Axillary LN | Probable: prior biopsy of hemorrhagic tumor mass |
| 22 | Large cell carcinoma/Lung | Mediastinal LN | Probable; prior lung cancer resection |
| 33 | Blastoma/Lung | Mediastinal LN | Possible; No mention of prior diagnostic procedures |
| 44 | GIST/Abdomen | Pericolic LN | Possible; No mention of prior diagnostic procedures |
| 55 | Melanoma/not stated | Cervical LN | Possible; No mention of prior diagnostic procedures |
| 66 | Papillary carcinoma/thyroid | Paratracheal LN | Probable; prior FNA biopsy |
| 77 | Cystic Renal cell carcinoma | Renal tumor | Probable; Multiple prior aspirations |
| 88 | Breast carcinoma | Axillary LN | Probable; Hemorrhagic breast biopsy at time of prior biopsy (similar to current case) |
| 99 | GIST/Stomach | Perigastric LN | Probable; prior resection |
| 10 current case | Breast carcinoma | Axillary LN | Definite. Catheter use documented. |
Although there is a widespread use of polyurethane catheters, the reports of pseudofungus structures are rare and limited to case reports. In the case reported here there was some difficulty with catheter placement and multiple attempts were made before the final catheter placement was made. It is postulated that during these attempted placements that the metal trocar scraped off fragments of polyurethane catheter, depositing them into the tissue. It is likely that in a simple uncomplicated catheter placement that only rare or no catheter fragments would be displaced into the tissue. This factor likely contributes to the rarity of finding pseudofungus structures.
In summary, pseudofungus structures were identified associated with a breast carcinoma that underwent needle biopsy with subsequent excessive hemorrhage requiring placement of a drainage catheter. Evidence is given that the pseudofungus structures identified in the draining axillary lymph node tissues represent fragments of the polyurethane catheter that were deposited in the breast tissues at the time of placement of the catheter and had migrated to the regional lymph nodes. The evidence includes both a comparison of these pseudofungus structures to fragments of polyurethane well documented in the literature and comparison of polyurethane catheter scrapings to the pseudofungus structures identified in the literature. In both of these comparisons the morphology of the polyurethane fragments is identical to the pseudofungus structures. In this era when many journals refuse to publish case reports, this publication should serve as an example of the contributions that a well thought out and investigated case report can make to the medical literature. This is the first definitive report identifying polyurethane catheter fragments as representing the true nature and etiology of pseudofungus structures.
- Connelly J, Cartwright J. Pseudofungi in a lymph node: A case report with energy dispersive x-ray elemental analysis. Arch Pathol Lab Med. 1991; 115: 1166-1168. PubMed: https://pubmed.ncbi.nlm.nih.gov/1747037/
- Teague MD, Tham KT. Hyphalike pseudofungus in the lymph node. Arch Pathol Lab Med. 1994; 118: 95-96. PubMed: https://pubmed.ncbi.nlm.nih.gov/8285843/ b
- Kim H, Koo JS, Shim H, et al. Pseudofungi associated with a granulomatous response in a lymph node: A case report. The Korean J of Pathol. 2004; 38: 64-67.
- Song ES, Kahn AG, Shin KK, Ro JY. Pseudofungi in pericolic lymph nodes. Arch Pathol Lab Med. 2005; 129: e97-e100. PubMed: https://pubmed.ncbi.nlm.nih.gov/15794699/
- Singh S, Manivel JC, Pambuccian SE. Pseudofungi: coral shapes and Bamboo sticks in lymph node sinuses. Int J Surg Pathol. 2010; 18: 68-69. PubMed: https://pubmed.ncbi.nlm.nih.gov/19748899/
- Lyapichev KA, Agarwal AA, Rosenberg AE, Chapman JR. Pseudofungi: A diagnostic pitfall. Int J Surg Pathol. 2016; 24: 528-531. PubMed: https://pubmed.ncbi.nlm.nih.gov/27106780/
- Nigdelioglu R, Biemer J, Pambuccian SE. Liesegang structures and pseudofungi in a cystic renal cell carcinoma. Diagnostic cytopath. 2018; 46: 888-891. PubMed: https://pubmed.ncbi.nlm.nih.gov/30043493/
- Bilani N, Elson L, Carlson D, Elimimian EB, Nahleh Z. Pseudofungi in an immunocompromised patient with breast cancer and COVID-19. Hindawi Case Reports in Medicine. 2020.
- Lau S, Williams D. PAS positive pseudofungi: a diagnostic pitfall. Pathology. 2021; 53: 542-543. PubMed: https://pubmed.ncbi.nlm.nih.gov/33218738/
- Zhou L, Pittmann W. Distributions organophosphate flame retardants (OPFRs) in 3 dust size fractions from homes and building material markets. Environmen Pollut. 2019; 245: 343-352. PubMed: https://pubmed.ncbi.nlm.nih.gov/30448504/