More Information
Submitted: December 20, 2021 | Approved: December 30, 2021 | Published: January 03, 2022
How to cite this article: Koeppen S, Hense J, Nolte KW, Joachim Weis. Immune-mediated neuropathy related to bortezomib in a patient with multiple myeloma. Arch Pathol Clin Res. 2022; 6: 001-004.
DOI: 10.29328/journal.apcr.1001028
Copyright License: © 2022 Koeppen S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Peripheral neuropathy; Immune mechanisms; Chemotherapy; Bortezomib; Multiple myeloma
Immune-mediated neuropathy related to bortezomib in a patient with multiple myeloma
Susanne Koeppen1*, Jörg Hense2, Kay Wilhelm Nolte3 and Joachim Weis3
1Department of Psychiatry and Psychotherapy, University Duisburg-Essen, Germany
2Department of Oncology, West German Tumor-Center, University Hospital Essen, Germany
3Institute of Neuropathology, RWTH University Aachen, Germany
*Address for Correspondence: Dr. Susanne Koeppen, Department of Psychiatry and Psychotherapy, University Duisburg-Essen, LVR-Klinikum Essen, Wickenburgstraße 23, 45147 Essen, Germany. Email: [email protected]
Treatment options in multiple myeloma (MM) based on novel agents are often limited by dose-related neurotoxicity. Bortezomib, a highly active reversible proteasome inhibitor, frequently causes peripheral neuropathy (PN). Bortezomib-induced PN (BIPN) is characterized by a length-dependent, sensory, axonal polyneuropathy (PNP) with predominant small fiber-affection. Following dose reduction or drug discontinuation, BIPN resolves within 3-4 months in the majority of patients. The pathophysiological mechanisms of BIPN are unclear. Rare cases of a severe demyelinating or mixed BIPN with prominent motor involvement have been attributed to autoimmune or inflammatory reactions. A case report, including nerve pathology, is presented of a 59-year-old man with stage III IgG-κ MM who was treated with bortezomib on the occurrence of progressive disease. After the fourth cycle, he developed a painful distal symmetric sensory PNP followed by gait instability and muscle weakness increasing over 3 months despite early cessation of bortezomib.
Neurological examination revealed a distal flaccid tetraparesis mainly of the lower limbs with sensory loss and severe ataxia, electrophysiological features of a mixed axonal-demyelinating PNP, and pathomorphological evidence of neuritis. Steroid treatment was initiated, and partial recovery of the neurological symptoms within 6 months was observed. While a neurotoxic effect may explain the initial distal sensory disturbances, the worsening of neurological dysfunction after bortezomib withdrawal and the clinical pattern with steroid-responsive muscle weakness predominantly of the legs are consistent with an immune-mediated mechanism. This is in line with the sural nerve biopsy findings. Toxic BIPN followed by an immune-mediated BIPN in the same patient has not been reported before.
The prevalence of PN in MM at the time of diagnosis is not well documented. Assessment of PN using the National Cancer Institute Common Toxicity Criteria of Adverse Events (NCI-CTCAE) grading scale revealed a sensory neuropathy in 20% of previously untreated patients [1]. Less frequently, MM-associated PN is the motor-predominant or mixed sensorimotor. On the other hand, treatment-emergent PN has become a major clinical problem. Bortezomib, a boronic acid dipeptide, is a selective and reversible 26S proteasome inhibitor demonstrating significant clinical response in MM patients with otherwise refractory or rapidly advancing disease. It has been approved by the US Food and Drug Administration (FDA) in 2003 as a monotherapy for progressive MM. Nowadays it is used either as a single agent or in combination with other agents [2]. Its mechanism of action in neoplastic cells is the inhibition of nuclear factor kappa B (NFκB), a transcription factor involved in cell survival and proliferation [3]. One of the common side effects associated with bortezomib therapy is PN. The neuropathy may be caused by the direct toxic effect of bortezomib or through an immunologically mediated process [4]. The incidence of neuropathy induced by bortezomib is around 30% - 60%. Although the neurotoxic mechanisms are not completely understood, experimental studies suggest that aggresome formation, endoplasmic reticulum stress, mitotoxicity, inflammatory response, and DNA damage could contribute to this neurotoxicity [5]. BIPN is dose-limiting toxicity that often requires adjustment of treatment and affects a patient’s prognosis and quality of life [3]. In the majority of cases, BIPN is manifesting as sensory-predominant and painful axonal neuropathy but a demyelinating neuropathy is also described primarily based on electrodiagnostic findings and rarely confirmed by nerve biopsy [6]. Data from animal models showing an increase in pro-inflammatory cytokine expression and changes in immune signaling pathways support the hypothesis that neuroinflammation is one of the major mechanisms underlying chemotherapy-induced peripheral neuropathy (CIPN). There is, however, the limited evidence available from human studies and it remains unclear whether neuroinflammatory responses are the cause of neuropathy or a bystander effect of the chemotherapy [7]. In the mouse model, morphological changes due to bortezomib treatment were found in the spinal cord, dorsal roots, dorsal root ganglia (DRG) and peripheral nerves. An in vitro model of BIPN using rat dorsal root ganglia neuronal cultures demonstrated that bortezomib induces an alteration in microtubules and axonal transport [8]. In patients with chronic painful BIPN, persistent and severe impairment of Aβ, Aδ, and C fibers was detected. Furthermore, a loss of both epidermal nerve fibers and Meissner’s corpuscles was found [9]. The unusual case reported here is characterized by a progressive tetraparesis mainly of the lower limbs developing after the onset of typical toxic BIPN despite discontinuation of bortezomib.
A 59-year-old man who was diagnosed with stage III IgG-κ MM in March 2006 attained a very good partial remission with 4 chemotherapy cycles consisting of vincristine, doxorubicin, and dexamethasone (VAD) followed by cyclophosphamide and 2 cycles of high-dose melphalan with autologous peripheral blood stem cell support. On the occurrence of progressive disease 3 years later, bortezomib was started in November 2009 at the standard dose (1.3 mg/m2).
After the fourth cycle in February 2010 (total dose 6.1 mg) the patient began to complain of numbness and painful paraesthesias at the hands and feet. Although bortezomib was discontinued, he developed gait instability and muscle weakness increasing over 3 months.
Neurological examination revealed a distal mild reduction of strength at the upper limbs (MRC 4/5), a mainly distal flaccid paraparesis of the lower limbs (MRC 0-2/5), absent ankle reflexes, muscle atrophy, bilateral anesthesia and analgesia with stocking distribution, distal loss of vibratory sensation and severe sensory ataxia.
Nerve conduction studies (NCS) displayed a mixed axonal-demyelinating PNP without conduction blocks. Needle electromyography (EMG) demonstrated active denervation changes in distal muscles of the lower limbs. Serological tests were negative for antibodies against myelin-associated glycoprotein (anti-MAG) and vasculitis parameters. Cerebrospinal fluid (CSF) analysis showed a moderate increase in protein content with normal cell count. There was no intraspinal MM manifestation on magnetic resonance imaging (MRI). Left sural nerve biopsy demonstrated an advanced focally accentuated axonopathy with endoneurial edema, clusters of CD68-immune-reactive macrophages, slightly increased cytotoxic T-lymphocytes and scarce regeneration activity. The patient was treated with methylprednisolone administered at 0.5 g intravenously for 3 days and then at a gradually reduced dose followed by dexamethasone 20 mg weekly. Within 6 months, partial recovery of the neurological symptoms occurred, and the patient regained the ability to walk using a stick. Three months later, he complained of right inguinal pain. On examination, he presented with distal hyporeflexia of the lower limbs, bilateral paresis of foot extensors (MRC 4/5) as well as toe extensors and flexors (MRC 3- 4/5), hypoesthesia and dysaesthesia of the fingers and feet and somatosensory ataxia of the legs. Electrophysiological testing showed a predominantly axonal sensorimotor neuropathy affecting both the distal and proximal parts of the peripheral nerves mainly of the lower limbs. In parallel, serum IgG level was rising, and infiltration of the right os pubis was detected by MRI. Unfortunately, the patient died from septicemia in February 2012.
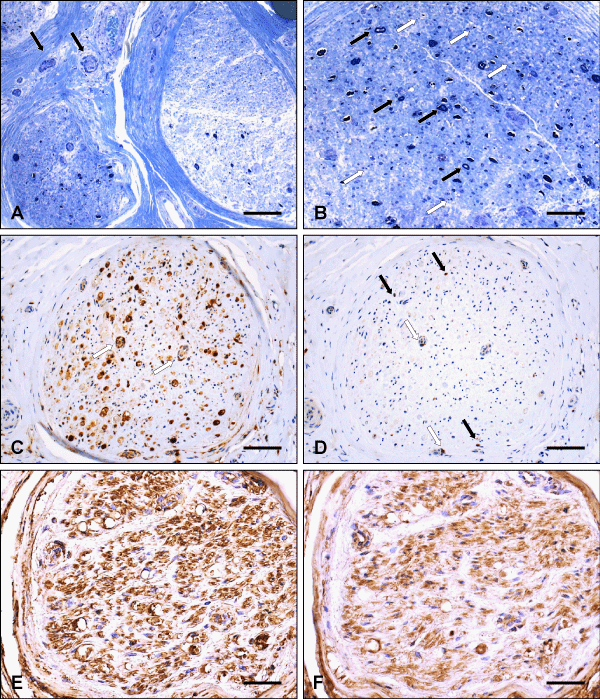
Histological analysis of sural nerve biopsy showed severe axonal loss unevenly distributed throughout the nerve fascicles and from fascicle to fascicle (Figure 1). Marked endoneurial edema was seen, mainly beneath the perineurium. Further-more, some mononuclear inflammatory cells were detected around small epineurial vessels, without vessel wall destruction. Several acutely degenerating nerve fibers, as well as myelin debris, was present, only a few myelinated nerve fibers were preserved. No hypertrophic changes (onion bulb formation), demyelinated axons, or regenerative groups were visible. CD68-immunostaining demonstrated numerous macrophages invading the endoneurium of a nerve fascicle, being focally clustered, e.g. around endoneurial vessels. CD8-immunohistochemical staining showed cytotoxic T- lymphocytes in the endoneurial compartment, some of them closely related to vessels. Within nerve fascicles, diffuse immunohistochemical kappa and lambda staining without clear preference of kappa over lambda was found after incubation with antisera to immunoglobulin light chains.
Figure 1: Histological analysis of the sural nerve biopsy. (A) Two adjacent nerve fascicles showing severe axonal loss which is unevenly distributed throughout the fascicles and from fascicle to fascicle. Note some mononuclear inflammatory cells around small epineurial vessels (arrows) without vessel wall destruction (resin embedded tissue, semithin section, toluidine blue). (B) Semithin transverse section of a nerve fascicle with only a few myelinated nerve fibers preserved (black arrows), several acutely degenerating nerve fibers as well as myelion debris (white arrows). No hypertrophic changes (onion bulb formation), demyelinated axons or regeneration groups are seen (toluidine blue stain). (C) Numerous macrophages invading the endoneurium of a nerve fascicle, being focally clustered, e. g. around endoneurial vessels (arrows) (Paraffin section, CD68-immunostaining). (D) Cytotoxic T-lymphocytes (arrows) in the endoneurial compartment of a nerve fascicle (same as in C), sometimes closely related to vessels (white arrows) (immunohistochemical staining for the CD8 T cell surface antigen). (E, F) Mainly diffuse staining of largely equal intensity within the same nerve fascicle after incubation with antisera to immunoglobulin light chains (immunohistochemical staining for kappa, E, and lambda, F). Scale bar: (A, C, D) 90 µm, (B, E, F) 45 µm.
The case presented here illustrates a rare form of BIPN with the dose-independent manifestation of severe motor signs, deterioration despite cessation of bortezomib and responsiveness to steroids suggesting an immunogenic mechanism. There were no symptoms or signs of peripheral neuropathy prior to the treatment with bortezomib. Particularly the clinical course with progressive tetraparesis shortly after bortezomib treatment makes peripheral neuropathy due to multiple myeloma very unlikely. BIPN occurs mainly in two forms: the first is a toxic predominantly sensory neuropathy, which is much more common, characterized by gradual onset, distal symmetric distribution, small fiber affection, axonal degeneration and remission within 3 to 4 months after withdrawal of bortezomib in the majority of patients having used a dose-modification guideline [10]. This dose-limiting peripheral neurotoxicity can significantly be reduced also by subcutaneous instead of intravenous administration of bortezomib [11]. The second form of BIPN is immune-mediated PN, which is less common, characterized by early-onset, prominent motor involvement, and response to steroids and intravenous immunoglobulin has [4]. In a rat model, the application of high-dose intravenous immunoglobulins (IVIG) was able to significantly reduce bortezomib-induced heat and mechanical allodynia. Moreover, IVIG administration was very effective in reducing infiltration in peripheral nerves of macrophages with the M1, pro-inflammatory phenotype. Results suggest a prominent role of neuroinflammation in BIPN and that IVIG might be considered as a possible safe and effective therapeutic option [12]. Recently, Xu, et al. published a patient with stage III MM-λ who complained of intense burning sensation and numbness in the lower limbs and hands associated with the absence of knee and ankle reflexes two weeks after the second course of bortezomib. He was diagnosed with Guillain-Barré syndrome based on albuminocytologic dissociation in CSF analysis and pathologic changes in nerve conduction studies. Following high-dose IVIG treatment he experienced relief, and all symptoms had fully resolved at 6-month follow-up [13]. A few cases of a rapidly evolving predominantly motor or unusual sensorimotor neuropathy under treatment with bortezomib suggesting a causal relationship have been reported so far.
Mauermann, et al. described a patient with MM who developed a severe subacute motor-predominant polyradiculoneuropathy with electrophysiological features of multiple conduction blocks, increased CSF protein and pathological evidence of peripheral nerve micro vasculitis following bortezomib treatment. The clinical course was characterized by further progression of the neuropathy despite bortezomib discontinuation, temporary stabilization with IVIG, lacking response to steroids and improvement after 5 cycles of cyclophosphamide [14]. A series of 5 MM patients reported by Ravaglia, et al. showed a bortezomib-associated neuropathy with prominent motor involvement, electrophysiological findings consistent with demyelinating or mixed polyneuropathy, CSF signs of inflammation, worsening neurological dysfunction after the last administration of bortezomib and positive response to immune treatments, either steroids or IVIG [15]. Jeter and Kang described a patient who has diagnosed with IgG kappa MM and started on therapy with single-agent bortezomib given at 1.3 mg/m2 intravenously weekly. During approximately the third cycle of bortezomib treatment, the patient started having severe pain in both legs as well as some mild paresthesias and muscle spasms. His chemotherapy was stopped. He also developed progressively worsening bilateral lower extremity weakness. Within one week, the patient was unable to walk and required the assistance of a wheelchair for mobility. There was electrophysiological evidence of widespread severe sensorimotor peripheral neuropathy. The patient underwent plasma exchange five times. After the second exchange, he was able to ambulate with a walker. However, his condition subsequently worsened and prednisone 40 mg PO daily was initiated. Within one to two days, his motor strength started to improve. Two months later, prednisone was gradually tapered. At the time of discontinuation of prednisone, he was able to ambulate with a cane and subsequently his performance status returned to his status prior to bortezomib treatment. He achieved complete remission from MM [4]. Another patient with MM and bortezomib-induced inflammatory neuropathy published by Saifee, et al. experienced pain in both calves, numbness and tingling in his feet and progressive leg weakness reaching a plateau after 2 months followed by spontaneous improvement with independent walking 9 months later. NCS demonstrated asymmetrical axonal sensorimotor neuropathy with acute denervation changes apparent in needle EMG. Superficial peroneal nerve biopsy revealed severe axonal loss and perivascular inflammation [16]. Schmitt, et al. describe a presumably bortezomib-triggered inflammatory autoimmune neuropathy mainly affecting the left brachial plexus and gradually resolving following application of IVIG. The identification of an inflammatory autoimmune neuropathy associated with bortezomib is a rare but important complication. The authors recommend an extensive neurological examination in patients who develop severe or unusual sensory or motor deficits under therapy with bortezomib, in order to differentiate autoimmune from toxic neuropathies, as therapeutic strategies differ for each [17].
In conclusion, the findings in our patient are different from previously reported cases in two essential aspects: first, in contrast to the case described by Mauermann, et al. sural nerve biopsy provided no clear diagnostic evidence of vasculitis. Similar to the histological changes reported by Saifee, et al., some perivascular inflammatory cells were detected in our case. Immunohistochemical analysis showed diffuse kappa and lambda staining within nerve fascicles reflecting the diffuse presence of immunoglobulin suggesting a polyclonal origin. Second, a manifestation of an immune-mediated BIPN subsequent to typical toxic BIPN in the same patient has not been published before. While a neurotoxic effect may explain the sensory symptoms, in the beginning, the worsening of neurological dysfunction after bortezomib discontinuation and the clinical pattern with response to steroid therapy are consistent with an immune-mediated polyneuritis. This was confirmed by pathomorphological findings.
Therapeutic response to methylprednisolone was observed being in line with nerve pathology suggesting an immunogenic mechanism.
- Richardson PG, Xie W, Mitsiades C, Chanan-Khan AA, Lonial S, et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009; 27: 3518-3525. PubMed: https://pubmed.ncbi.nlm.nih.gov/19528374/
- Meregalli C. An Overview of Bortezomib-Induced Neurotoxicity. Toxics. 2015; 3: 294-303. PubMed: https://pubmed.ncbi.nlm.nih.gov/29051465/
- Ale A, Bruna J, Calls A, Karamita M, Haralambous S, et al. Inhibition of the neuronal NFkappaB pathway attenuates bortezomib-induced neuropathy in a mouse model. Neurotoxicology. 2016; 55: 58-64. PubMed: https://pubmed.ncbi.nlm.nih.gov/27211850/
- Jeter A, Kang Y, Immune modulation therapy in the management of bortezomib-induced peripheral neuropathy. Exp Hematol Oncol. 2012; 1: 20. PubMed: https://pubmed.ncbi.nlm.nih.gov/23211009/
- Ale A, Bruna J, Navarro X, Udina E. Neurotoxicity induced by antineoplastic proteasome inhibitors. Neurotoxicology. 2014; 43: 28-35. PubMed: https://pubmed.ncbi.nlm.nih.gov/24525285/
- Thawani SP, Tanji K, De Sousa EA, Weimer LH, Brannagan 3rd TH. Bortezomib-associated demyelinating neuropathy--clinical and pathologic features. J Clin Neuromuscul Dis. 2015; 16: 202-209. PubMed: https://pubmed.ncbi.nlm.nih.gov/25996966/
- Lees JG, Makker PGS, Tonkin RS, Abdulla M, Park SB, et al. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer. 2017; 73: 22-29. PubMed: https://pubmed.ncbi.nlm.nih.gov/28104535/
- Staff NP, Podratz JL, Grassner L, Bader M, Paz J, et al. Bortezomib alters microtubule polymerization and axonal transport in rat dorsal root ganglion neurons. Neurotoxicology. 2013; 39: 124-131. PubMed: https://pubmed.ncbi.nlm.nih.gov/24035926/
- Boyette-Davis JA, Cata JP, Zhang H, Driver LC, Wendelschafer-Crabb G, et al. Follow-up psychophysical studies in bortezomib-related chemo neuropathy patients. J Pain. 2011; 12: 1017-1024. PubMed: https://pubmed.ncbi.nlm.nih.gov/21703938/
- Richardson PG, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009; 144: 895-903. PubMed: https://pubmed.ncbi.nlm.nih.gov/19170677/
- Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomized, phase 3, non-inferiority study. Lancet Oncol. 2011; 12: 431-140. PubMed: https://pubmed.ncbi.nlm.nih.gov/21507715/
- Meregalli C, Marjanovic I, Scali C, Monza L, Spinoni N, et al. High-dose intravenous immunoglobulins reduce nerve macrophage infiltration and the severity of bortezomib-induced peripheral neurotoxicity in rats. J Neuroinflammation. 2018; 15: 232. PubMed: https://pubmed.ncbi.nlm.nih.gov/30131066/
- Xu YL, Zhao WH, Tang ZY, Li ZQ, Long Y, et al. Guillain-Barre syndrome in a patient with multiple myeloma after bortezomib therapy: A case report. World J Clin Cases. 2019; 7: 2905-2909. PubMed: https://pubmed.ncbi.nlm.nih.gov/31616710/
- Mauermann ML, Blumenreich MS, Dispenzieri A, Staff NP. A case of peripheral nerve microvasculitis associated with multiple myeloma and bortezomib treatment. Muscle Nerve. 2012; 46: 970-977. PubMed: https://pubmed.ncbi.nlm.nih.gov/23225391/
- Ravaglia S, Corso A, Piccolo G, Lozza A, Alfonsi E, et al. Immune-mediated neuropathies in myeloma patients treated with bortezomib. Clin Neurophysiol. 2008; 119: 2507-2512. PubMed: https://pubmed.ncbi.nlm.nih.gov/18829381/
- Saifee TA, Elliott KJ, Rabin N, Yong KL, D'Sa S, et al. Bortezomib-induced inflammatory neuropathy. J Peripher Nerv Syst. 2010; 15: 366-368. PubMed: https://pubmed.ncbi.nlm.nih.gov/21199108/
- Schmitt S, Goldschmidt H, Storch-Hagenlocher B, Pham M, Fingerle-Rowson G, et al. Inflammatory autoimmune neuropathy, presumably induced by bortezomib, in a patient suffering from multiple myeloma. Int J Hematol. 2011; 93: 791-794. PubMed: https://pubmed.ncbi.nlm.nih.gov/21553020/