More Information
Submitted: 07 January 2020 | Approved: 13 January 2020 | Published: 14 January 2020
How to cite this article: Lombard CM. Uterine precursor lesions in patients with incidental nodal lymphangioleiomyomatosis: A report of 4 cases. Arch Pathol Clin Res. 2020; 4: 001-004.
DOI: 10.29328/journal.apcr.1001016
ORCiD: orcid.org/0000-0001-8384-7511
Copyright License: © 2020 Lombard CM. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Nodal lymphangioleiomyomatosis; Uterine precursor lesion; Pathogenesis; Pathology
Uterine precursor lesions in patients with incidental nodal lymphangioleiomyomatosis: A report of 4 cases
Charles M Lombard*
M.D, Department of Pathology, El Camino Hospital, Associate Clinical Professor of Pathology, Stanford University, Room GC #431, Mountain View, CA 94040, USA
*Address for Correspondence: Charles M Lombard, M.D, Department of Pathology, El Camino Hospital, Associate Clinical Professor of Pathology, Stanford University, Room GC #431, Mountain View, CA 94040, USA, Tel: +650-940-7033; Fax: 650-966-9292; Email: [email protected]; [email protected]
Uterine sections from 6 patients with incidental nodal lymphangioleiomyomatosis (LAM) were examined for LAM lesions by screening these sections with cathepsin K immunohistochemistry (IHC) stains. The hysterectomy specimens were all concurrent with the lymph node dissections in which the nodal LAM was discovered. In 4 of 6 patients microscopic lesions of pre-LAM were identified and confirmed by IHC staining for HMB-45 and beta-catenin. All lesions were grossly inapparent and also inapparent by routine hematoxylin and eosin stains. Three variants of pre-LAM lesions were identified. None of the pre-LAM lesions had an associated lymphatic proliferation. It is proposed that these pre-LAM lesions gave rise to the incidental nodal LAM lesions. Furthermore, it is suggested that the absence of an associated lymphatic proliferation associated with these lesions may be a factor in the attenuated potential for spread and the only rare association of these nodal lesions with pulmonary LAM.
Incidental nodal lymphangioleiomyomatosis (LAM) has been well described in 3 recent series [1-3] and for the most part does not appear to be a harbinger of pulmonary LAM. However, the pathogenesis of these nodal lesions is not well described. In particular, it is not known whether these lesions arise de novo in the affected lymph nodes from smooth muscle in the lymph node capsules or whether the nodal LAM represents a metastasis. If these lesions represent metastases, the primary site for these lesions has not been identified. It is known that in patients with pulmonary LAM, that uterine and adnexal lesions of LAM can be identified and it has been proposed that these lesions are the primary site of the LAM cells that spread to the lungs [4]. It was my hypothesis that the primary site for LAM spread to the pelvic and para-aortic lymph nodes with incidental nodal LAM would likely be the same sites as the precursor lesions for pulmonary LAM, in particular the uterine myometrium. The objective of this study was to identify uterine precursor LAM lesions and describe their morphologic appearance(s). To this end, the surgical pathology files at El Camino Hospital were searched for cases of incidental nodal LAM involving pelvic and/or para-aortic lymph nodes where there was also a hysterectomy specimen. My hypothesis was that precursor LAM lesions would be identified that could give rise to the nodal LAM. If identified, it was also possible that there might be features that would explain the more attenuated potential for spread as compared to the lesions that result in pulmonary LAM.
Case selection
The case files of El Camino Hospital were searched for cases of nodal LAM. 7 cases were identified, however, only 6 of these cases had an associated hysterectomy specimen. These cases derive from 953 cases of gynecologic neoplasms (cervical, uterine, tubal and ovarian) in which pelvic and/or aortic lymph nodes were sampled. In addition, 6 control cases were identified from endometrial carcinoma resections whose regional lymph nodes showed no evidence of nodal LAM.
Histologic assessment
All slides from the cases identified with nodal LAM were reviewed and the diagnosis of nodal LAM confirmed. All cases of nodal LAM showed characteristic histologic features and showed positive staining for Cathepsin K and HMB-45. All sections of myometrium were examined from all 6 cases with nodal LAM for histologic evidence of a typical uterine LAM lesion. There were no histologically identifiable lesions in any of these cases on routine hematoxylin and eosin stains.
IHC studies
All sections of myometrium from the cases with confirmed nodal LAM were stained with cathepsin K (Cell Marque/Sigma antibody; RTU; Leica Bond 3 with ER1 retrieval protocol at 20 min), HMB-45 (Leica antibody; RTU; Leica Bond 3 with enzyme 1 retrieval at 5 min), beta-catenin (Leica antibody; RTU; Leica Bond 3 with ER1 retrieval protocol at 20 min), desmin (Leica antibody; RTU; Leica Bond 3 with ER2 retrieval protocol at 20 min), D2-40 (Leica antibody; RTU; Leica Bond 3 with ER2 retrieval protocol at 20 min). Representative sections of myometrium from each of the 6 control cases (without nodal LAM) were also stained with cathepsin K.
Clinical and pathologic features for the 6 identified cases of nodal LAM with an associated hysterectomy specimen including age, primary pathologic diagnosis for specimen, LN involvement, number of sections of uterus examined, number of uterine sections with lesions, and total number of lesions identified are displayed in table 1. Results of the immunohistochemical evaluation of the uterine sections are displayed in table 2.
| Table 1: Clinical features, # myometrial sections, # lesions detected. | ||||||
| Case # | Age | Primary pathologic diagnosis | LNs examined (# with LAM/total) |
# sections uterus/ # sections with pre-LAM lesions |
Total # of pre-LAM lesions | Sizes of pre-LAM lesions |
| 1* | 47 | FIGO 2 EM CA with superficial invasion; LNs negative | Pelvic (1/10) | 8/3 | 3 | 0.25-0.75 mm |
| 2 | 61 | EM serous CA with deep invasion; LNs positive | Pelvic (4/8) Aortic (3/4) |
3/1 | 1 | 3.5 mm |
| 3 | 64 | Endocervical CA with deep invasion; LNs negative | Pelvic (1/16) Aortic (0/19) |
6/3 | 3 | 0.25-0.50 mm |
| 4 | 57 | FIGO 1 EM CA with superficial invasion; LNs negative | Pelvic (3/15 Aortic (1/4) |
6/6 | > 40 | 0.25-1.7 mm Diffuse area: 12 x 8 mm |
| 5 | 46 | Clear cell CA left ovary; LNs negative | Pelvic (2/17) Aortic (0/7) |
4/0 | 0 | N/A |
| 6 | 38 | Squamous carcinoma cervix; LNs positive | Pelvic (1/18) | 5/0 | 0 | N/A |
| *Case 1 was the subject of a prior case report [5]. | ||||||
| Table 2: IHC staining patterns. | |||||
| Case # | Cathepsin K | HMB-45 | Beta-Catenin | Desmin | D2-40 |
| 1** | + clusters of spindle cells | + subset of lesions | Trace + | + | (-)* |
| 2 | + clusters of spindle cells | + | Strong + | + | (-)* |
| 3 | + rare clusters of epithelioid appearing cells |
+ epithelioid cells | Moderate + epithelioid cells |
+ | (-)* |
| 4 | + clusters of spindle cells; Area of diffuse positive Staining in a splayed pattern |
+ subset of Spindle lesions; + in diffuse splayed area |
Variable + In spindle areas moderate to strong; (-) in diffuse splayed area |
+ | (-)* |
| 5 | Negative | (-) | (-) | + (normal myometrium) | (-) normal |
| 6 | Negative | (-) | (-) | +(normal myometrium) | (-) normal |
| *D2-40 staining pattern appears normal without increased lymphatic proliferation associated with the precursor uterine LAM lesions **Case 1 was the subject of a prior case report [5]. |
|||||
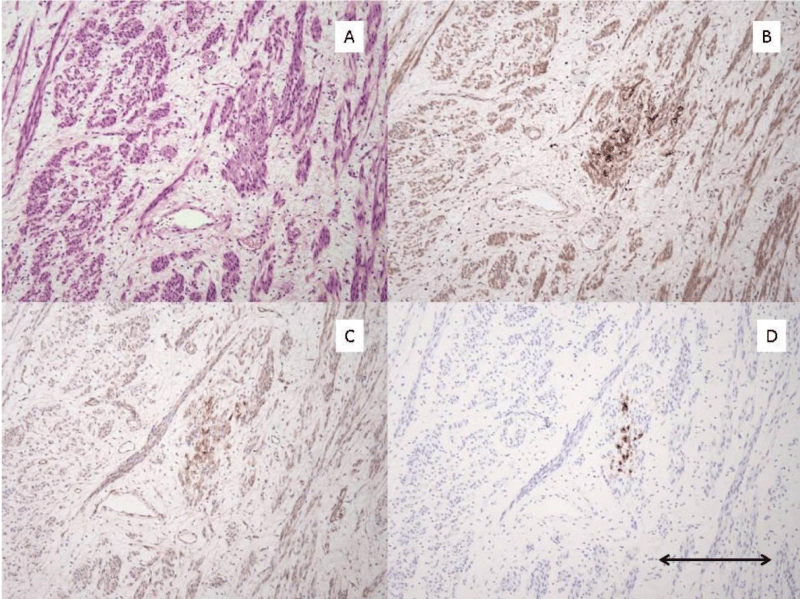
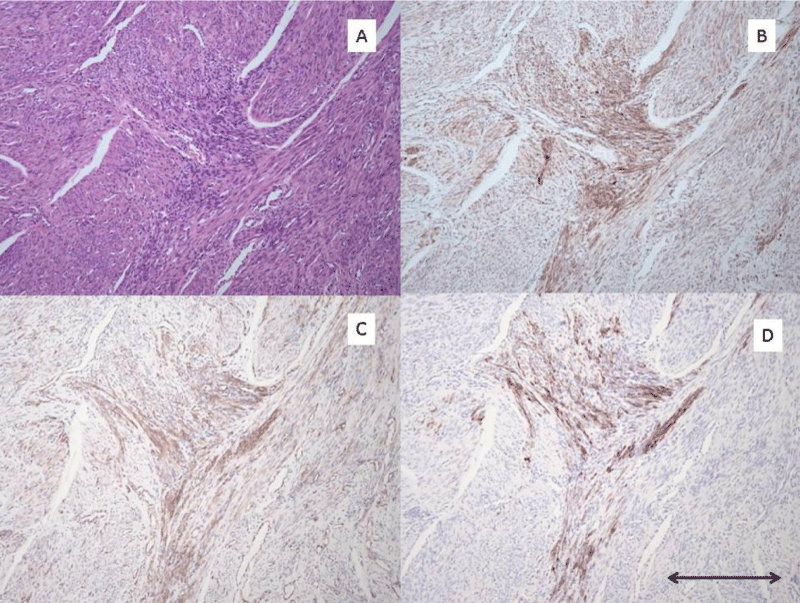
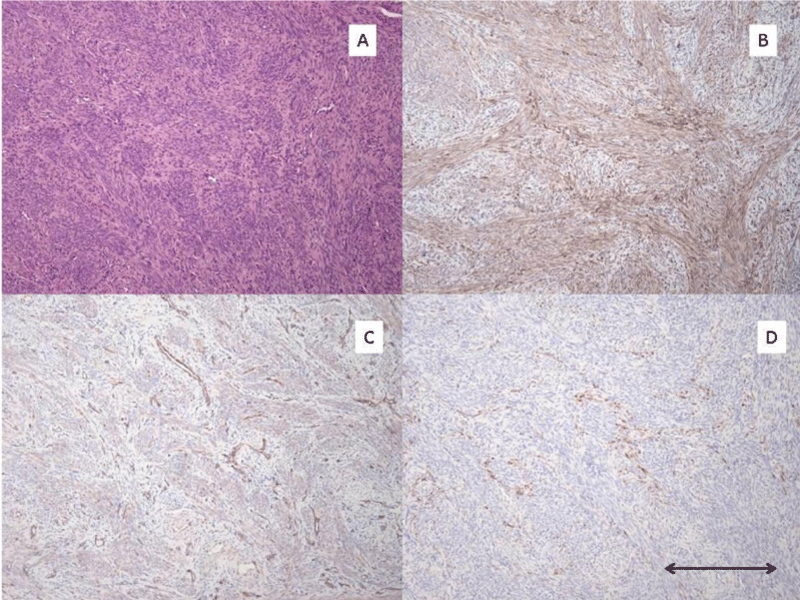
3 morphologic variants of pre-LAM lesions were identified. The first morphologic variant is one which may represent the earliest manifestation of these lesions. The lesion is composed of epithelioid appearing cells in small clusters which strongly express cathepsin K and HMB-45 (Figure 1). Although it is possible that these represent spindled cells cut on end, I suspect they represent the earliest morphologic manifestation of the mutated Mullerian smooth muscle cell before limited proliferation leads to the more spindle shaped forms of these cells. The second morphologic variant is the one I have found to be most common. In these lesions there is a proliferation of spindle-shaped altered smooth muscle cells which are present in small localized fascicles, and as they expand, take on a stellate appearance (Figure 2). The third morphologic variant, seen in one case, is characterized by a diffuse insinuation of the spindle-shaped pre-LAM cells in an area of normal myometrial smooth muscle cells (Figure 3). This lesion is most difficult to detect as the cathepsin K positive cells are spread out and more difficult to identify than when they occur in tight clusters. However, the HMB-45 in this area of diffuse insinuation was strong and readily identifiable in the altered smooth muscle cells. The beta catenin was negative in this area.
Figure 1: Pre-LAM uterine lesion pattern #1. A): H/E stain of area with Pre-LAM lesion composed of cluster of small epithelioid appearing cells. Lesional cells are inapparent on this stain. B): Cathepsin K IHC stain shows strong positive staining of the small cluster of epithelioid cells. C): Beta-catenin IHC stain shows moderate staining of the small cluster of epithelioid cells. D): HMB-45 IHC stain shows strong positive staining of the small cluster of epithelioid cells. All photomicrographs taken at 100x magnification. Scale bar in lower right corner measures 0.2 mm.
Figure 2: Pre-LAM uterine lesion pattern #2 A): H/E stain of area with pre-LAM lesion composed of spindle cells without distinct morphologic variance from surrounding uterine smooth muscle cells. B): Cathepsin K IHC stain with strong positive staining of the small stellate proliferation of pre-LAM cells. C): Beta-catenin IHC stain with moderate positive staining of the small stellate proliferation of pre-LAM cell. D): HMB-45 IHC stain with strong positive staining of the small stellate proliferaton of pre-LAM cells. All photomicrographs taken at 100x magnification. Scale bar in lower right corner measures 0.2 mm.
Figure 3: Pre-LAM uterine lesion pattern #3. A): H/E stain of pre-LAM lesion with a histologically inapparent diffuse insinuation with adjacent uterine smooth muscle cells. B): Cathepsin K IHC stain showing moderate positive staining of the pre-LAM cells dissusely insinuated with the normal myometrial smooth muscle cells. C): Beta-catenin IHC stain without staining of the pre-LAM cells with the diffuse insinuating growth pattern. D): HMB-45 IHC stain with moderate positive staining of the pre-LAM cells with the diffuse insinuating growth pattern. All photomicrographs taken at 100x magnification. Scale bar in lower right corner measures 0.2 mm.
The incidence of nodal LAM in this series is 0.73% (7/953 cases). This is remarkably similar to the 0.46% incidence that has been previously reported [3]. Incidental nodal LAM has limited potential for proliferation and spread with only one case of subsequent pulmonary LAM having been described [3]. I have previously reported one of the cases in this current series (case 1) of incidental nodal LAM where uterine precursor LAM lesions were identified in the uterus [5]. I have arbitrarily designated these proliferations as pre-LAM lesions because of the consistent absence of an associated lymphatic proliferation. This report further documents these pre-LAM lesions and further expands the spectrum of their appearances. It is important to note that all of these lesions are grossly inapparent and inapparent on routine H+E microscopy. In this study, the most sensitive marker for detection of these pre-LAM lesions is cathepsin K. HMB-45 and cytoplasmic beta-catenin staining can provide confirmatory evidence for the presence of the pre-LAM lesion. Beta-catenin was used as a report has indicated that LAM cells have positive staining for cytoplasmic beta-catenin [6]. However, neither of these markers are sufficiently sensitive to be used as a screening test for the lesions.
Because of the retrospective nature of this study, the number of myometrial sections for each case was limited. Nevertheless, in 4 of 6 cases evidence of these pre-LAM lesions were identified. In 6 control cases, without incidental nodal LAM, there was no evidence of these pre-LAM lesions identified. If additional sections were examined from the 2 negative cases, I suspect lesions of pre-LAM would have been identified. In one of that four cases I report, there were numerous pre-LAM lesions in all of the random sections of myometrium. However, in the remaining 3 cases, the number of lesions were quite limited and not present in every myometrial section. Because these are grossly invisible, without examination of myometrial sections of the uterus with an immunohistochemical stain for cathepsin K, these pre-LAM lesions will been missed.
I have identified 3 morphologic variants of pre-LAM lesions, although I believe all are related and have no special significance. I note them here to help facilitate identification of uterine pre-LAM lesions. The proliferations identified and described above have no morphologic features which reliably allow them to be identified in routine hematoxylin and eosin stained sections as they look indistinguishable from the surrounding normal myometrial smooth muscle cells. They are best identified by immunohistochemical staining for cathepsin K and in many cases can be confirmed by immunohistochemical staining for HMB-45 and cytoplasmic beta catenin. It is important to note that in all 4 cases there is no associated lymphatic proliferation. For this reason I have proposed the term pre-LAM to better describe them. In all well-developed LAM lesions including the uterus [4], the lymph nodes [2], and the lungs [7], there is a lymphatic proliferation associated with the altered smooth muscle proliferation. In particular, this is true for the uterine LAM lesions which have been described in patients with the tuberous sclerosis complex and pulmonary LAM. An alternative term for these pre-LAM lesions might be “microscopic melanomyocytic” proliferations. However, I prefer the term pre-LAM to underscore their relationship to LAM lesions. I propose that these uterine pre-LAM lesions give rise to the incidental nodal LAM by spread from the uterus to the regional lymph nodes. The absence of an associated lymphatic proliferation may be the reason for the apparent limited potential for extensive spread of the cells and the only rare association with pulmonary LAM. In this regard, the degree of lymphangiogenesis in pulmonary LAM has been correlated with the histologic severity of the disease [8].
Finally, I believe these additional cases of uterine pre-LAM lesions adds further support to my proposal that the precursor to the LAM cell is a Mullerian smooth muscle cell. Other investigators have identified LAM lesions in the female genital tract including the fallopian tubes and ovaries as well as the myometrium , which they have proposed to be precursor lesions for pulmonary LAM [4]. In the lesions I describe, the intimate admixture of the pre-LAM cells with normal smooth muscle cells of the uterus and essential histologic identity with normal smooth muscle cells, in my opinion, supports this concept.
In summary, 6 patients with incidentally discovered nodal LAM who also had an associated hysterectomy specimen, underwent a retrospect the examination of their uterine histologic sections with immunohistochemical stains for evidence of uterine lesions. In 4 of 6 patients, uterine lesions of pre-LAM were identified. It is likely that in the other 2 patients lesions were present but not sampled. I propose that these pre-LAM lesions are the primary site for the cells that spread to the regional lymph nodes and form incidental nodal LAM lesions. The absence of an associated lymphatic proliferation may be a factor in their limited potential for spread and the only rare association with pulmonary LAM.
Finally, I propose that the Mullerian smooth muscle cell is precursor cell for the LAM cell.
- Schoolmeester JK, Park KJ. Incidental nodal lymphangioleiomyomatosis is not a harbinger of pulmonary lymphangioleiomyomatosis: a study of 19 cases with evaluation of diagnostic immunohistochemistry. Am J Surg Pathol. 2015; 39: 1404-1410. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26135558
- Rabban JT, Firetag B, Sangoi AR, Post MD, Zaloudek CJ. Incidental pelvic and para-aortic lymph node lymphangioleiomyomatosis detected during surgical staging of pelvic cancer in women without symptomatic pulmonary lymphangioleiomyomatosis or tuberous sclerosis complex. Am J Surg Pathol. 2015; 39: 1015-1025. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25786086
- Kuno I, Yoshida J, Shimizu H, Uehara T, Uno M, et al. Incidental lymphangioleiomyomatosis in the lymph nodes of gynecologic surgical specimens. Eur J Ob Gynec Reprod Biol. 2018; 231: 93-97. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30336310
- Hayashi T, Kumasaka T, Mitani K, Terao Y, Watanabe M, et al. Prevalence of uterine and adnexal involvement in pulmonary lymphangioleiomyomatosis: a clinicopathologic study of 10 patients. Am J Surg Pathol. 2011; 35: 1776-1785. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22020043
- Lombard CM. Microscopic precursor lesions of uterine lymphangioleiomyomatosis associated with incidental nodal lymphangioleiomyomatosis. A case report and discussion of pathogenesis. Hum Pathol Case Rep (in press).
- Flavin RJ, Cook J, Fiorentino M, Bailey D, Brown M, et al. Beta-Catenin is a useful adjunct immunohistochemical marker for the diagnosis of pulmonary lymphangioleiomyomatosis. Am J Clin Pathol. 2011; 135: 776-782. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21502434
- Kumasaka T, Seyama K, Mitani K, Sato T, Souma Sm et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004; 28: 1007-1016. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15252306
- Gyure KA, Hart WR, Kennedy AW. Lymphangioleiomyomatosis of the uterus associated with tuberous sclerosis and malignant neoplasia of the female genital tract: a report of two cases. Int J Gynecol Pathol. 1995; 14: 344-351. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8598338