Case Report
A great mimicker of Bone Secondaries: Brown Tumors, presenting with a Degenerative Lumber Disc like pain

Zuhal Bayramoglu1*, Ravza Yılmaz1 and Aysel Bayram2
1Department of Radiology, Istanbul Medical Faculty, Istanbul University, Capa, Fatih, 34093, Istanbul, Turkey
2Department of Pathology, Istanbul Medical Faculty, Istanbul University, Capa, Fatih, 34093, Istanbul, Turkey
*Address for Correspondence: Dr. Zuhal Bayramoglu, Department of Radiology, Istanbul Medical Faculty, Istanbul University, Capa, Fatih, 34093, Istanbul, Turkey, Tel: +90-212- 414-20-00, Fax: +90-212-631-07-28; Email: [email protected]
Dates: Submitted: 06 June 2017; Approved: 14 July 2017; Published: 17 July 2017
How to cite this article: Bayramoglu Z, Yılmaz R, Bayram A. A great mimicker of Bone Secondaries: Brown Tumors, presenting with a Degenerative Lumber Disc like pain. Arch Pathol Clin Res. 2017; 1: 018-023. DOI: 10.29328/journal.apcr.1001004
Copyright License: © 2017 Bayramoglu Z et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Brown tumor; Multiple; Iliac bone; Radius; Sacrum; Magnetic resonance imaging; Computed tomography; Sacroiliitis; Radiculopathy; Hyperparathyroidism
ABSTRACT
This report presents an adult patient suffering from sacroiliitis like low back pain, lumbosacral radiculopathy and elbow swelling. Multimodality imaging revealed multiple lytic bone lesions located in supra acetabular iliac bone, sacrum, and distal end of radius. Painful numerous lesions due to the extension to the articular surfaces are not expected for Brown tumors. Less than ten cases with multiple Brown tumor due to primary hyperparathyroidism has been reported. Although Brown tumors are mostly diagnosed incidentally, this case would awake the physicians about rheumatological symptoms in the presentation of Brown tumors. Since Brown tumors are non-touch bone lesions that are expected to regress after parathyroid adenoma removal, it is important to distinguish Brown tumors from the giant cell tumors.
CASE PRESENTATION
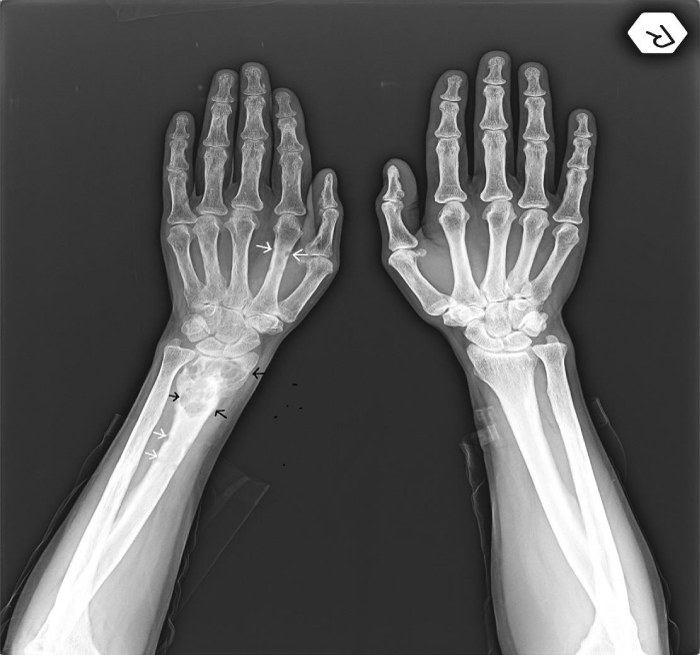
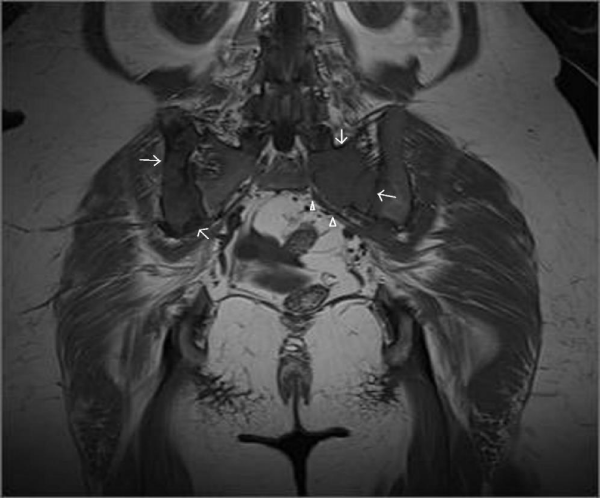
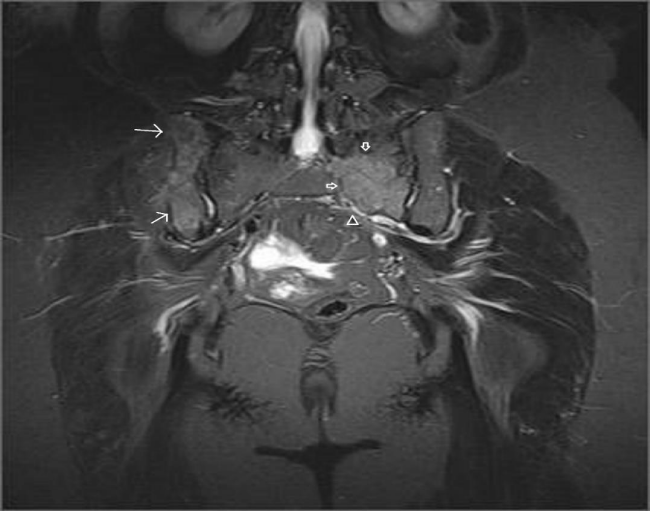
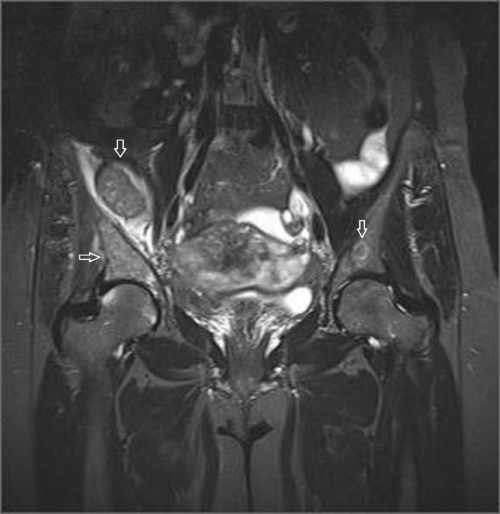
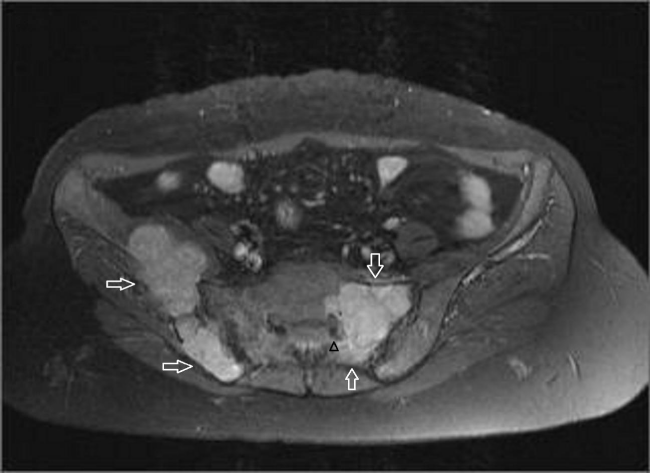
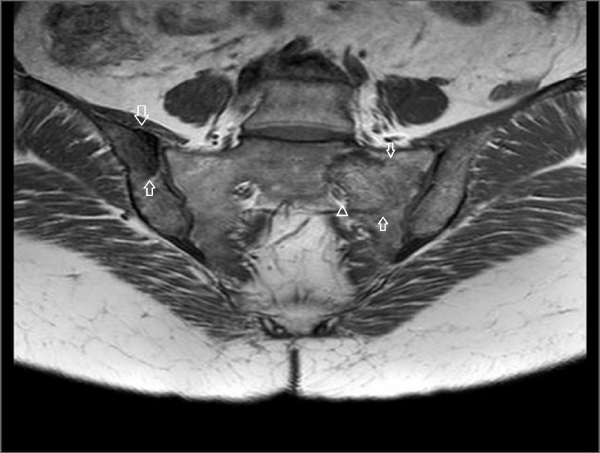
A 45-year-old female referred to physical therapy and rehabilitation clinic with a complaint of progressive low back and hip pain for two years radiating along the posterior thigh, posterolateral aspect of her left leg and lateral aspect of left foot that increases by walking especially with wider steps and decreases with resting considering lumbosacral radiculopathy. Bilateral FABER (flexion, abduction, external rotation), compression and distraction tests were performed and confirmed bilateral sacroiliac joint pain. The patient reported to ignore her left hand because of painful elbow movements besides swelling. Multifocal painful joint movements with proximal muscle weakness indicated further radiologic investigation. Lytic, expansile masses close to the bilateral sacroiliac joints arising from the left half of the sacrum and right iliac bone, and the another one located in the supra-acetabular region were depicted on conventional radiography (CR). Also hand and wrist radiograph demonstrated a mass located in the distal end of radius adjacent to radiocarpal joint surface extending to subchondral bone with expansile remodeling having similar radiological appearances with the tumors in pelvic bones figure 1. Areas of tunnel shaped radiolucency in the second metacarpal and radius cortex were depicted figure 1. A sclerotic rim on CR was not existing for the reported masses and any history of malignancy was not present. Pelvic magnetic resonance imaging [MRI] was provided to clarify the cause of radiculopathy. The masses were heterogeneous and hypointense on T1-weighted (T1W) MRI figure 2. They showed heterogeneous and mildly hyperintense signal on T2-weighted (T2W) MRI figure 3,4. Heterogeneous and intense contrast enhancement on fat-saturated T1W MRI figure 5. All of the masses were expansile and extending to closest joint surface. The one located on the left hemisacrum obliterated the left L5-S1 and S1-S2 neural foramina and compressed the adjacent left L5 and S1 sacral nevre roots figure 5. The mass on the right iliac bone was arising to sacroiliac joint surface associated with the sacroiliac joint dysfunction and the other mass was arising to acetabular surface associated with hip pain. Hyperparathyroidism was investigated through biochemical analysis and a high level of calcium [11.8mg/dl] and parathormone [PTH:674pg/ml], with an increased ALP level were depicted. Laboratory outcomes found a diagnosis of primary hyperparathyroidism and a parathyroid adenoma was depicted at the lower aspect of the left paratracheal region by ultrasound and confirmed after surgical excision figure 6.
Figure 1: Elbow roentgenogram: Arrow shows a radiolucent lesion of the radius distal metaphysis without significant soft tissue expansion. Arrow head shows subperiosteal resorption foci and scalloping.
Figure 5: Post contrast (gadolinium-enhanced) axial images show homogeneously enhancing tumor lining to the left sacroiliac joint surface and narrowing of the left sacral neural foramina. Left hemisacrum involvement is suspicious for sacral nerve compression due to perineural obliteration.
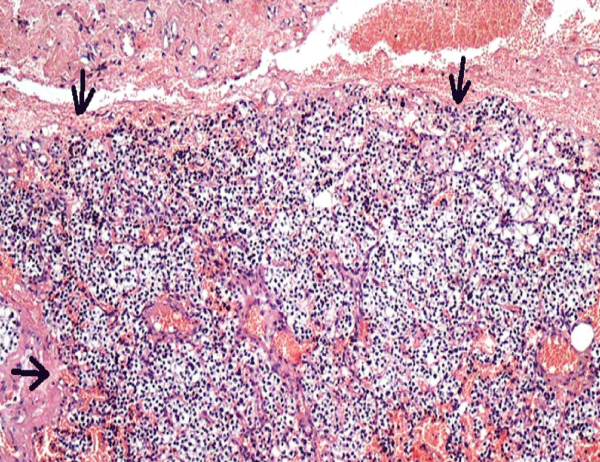
Figure 6: The tumor was surrounded by a thin fibrous capsule of connective tissue. Hematoxylin and eosin, 100X].
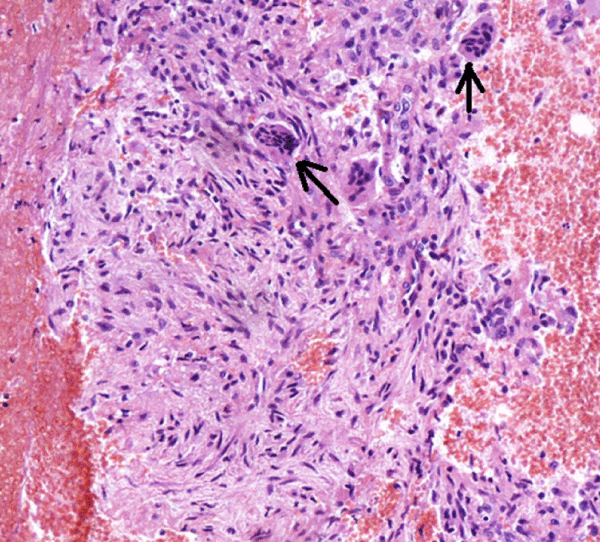
CT-guided percutaneous bone tru-cut biopsy was performed after obtaining written informed consent from the patient. Histopathology revealed fibroblastic tissue containing numerous osteoclast-like giant cells figure 7. The PTH level was 35 pg/ml and the calcium level was 8.5mg/dl a few days after surgery. After adenoma removal, whole masses totally regressed and massive sclerotic bone formation was observed on MRI within a year figure 8 and confirmed the diagnosis of BTs.
Figure 7: Fibroblastic tissue containing numerous osteoclast-like giant cells (Hematoxylin and eosin, 200X).
Figure 8: Complete regression of the tumor in the same patient. There is neither a finding of oedema on the STIR [short-tau inversion recovery] sequence [A] nor residual tumor on the contrast-enhanced T1W image after six months from the parathyroid adenoma excision.
DISCUSSION
Based on radicular pain in this adult most common cause of radiculopathy is known to caused by degenerative disc diseases. Also, contralateral painful sacroiliac joint movement could consider osteitis condensans ilii in an adult female patient presented with sacroiliitis like pain. Thus, initial diagnostic tool could be conventional radiograph in order to demonstrate osteophytic processes and triangular shaped sclerosis adjacent to the the articular surface of the iliac bone. Despite these common degenerative processes, we discuss multiple Brown tumors due to primary hyperparathyroidism as a cause of radiculopathy and sacroiliitis like pain.
The frequency of Brown Tumor (BTs) are reported to be 3% in primary and 2% in secondary hyperparathyroidism [1]. Brown tumors are the less common muskuloskeletal presentation of primary hyperparathyroidism [2]. Most common involved parts of the body with BTs were reported to be facial bones [mandible, maxilla], sternum, ribs, phalanx, and femur [3]. Although any bone could be affected by BTs, involvement of the sacral spine is reported to be extremely rare [4]. Multiple Brown tumors in the setting of primary hyperparathyroidism is extremely rare [5] and could be misdiagnosed as metastasis or giant cell tumors (GCTs), that seen in association with Paget disease. The closest differential diagnosis of giant cell tumor was excluded with elevated parathormone and calcium levels. Arising from the sacrum, involving multiple bones, and presenting with radiculopathy and sacroiliitis like pain are expected for giant cell tumor but such a clinical and radiological entity has not been demonstrated in terms of BT presentation.
Differential diagnosis of multiple osteolytic masses on computed tomography includes bone cysts, giant-cell granuloma, GCTs, multiple myeloma, lymphoma aneurysmal bone cysts, BTs and chordoma especially when they are located in sacrum [6]. Most of the benign bone lesions demonstrate a sclerotic margin and multiple myeloma, metastasis and GCTs do not have this finding as well as the case presented.
Bone lypmphomas commonly involve the pelvic bones and femur and commonly demonstrate a permeative bone destruction pattern instead of a well marginated expansile lytic mass [7]. Chordomas of the spine are rare lesions but they are the most common primary spinal malignancy in adults after lymphoproliferative diseases [8]. Cordomas are often well-circumscribed lytic expansile masses which may include irregular intratumoral calcifications due to sequestra of normal bone, may present marginal sclerosis and involve more than one vertebral segment. Thus, they have a strong potential of a possible differential diagnosis. Exantric and cortical location in absence of a history of malignancy could consider BTs rather than metastasis, myeloma and lymphoma. MRI is a useful imaging modality in order to detect the cystic and solid components, the hemosiderin deposits based on susceptibility artifacts, enhancing reactive fibrovascular tissue and the cause of neurologic disorder as well. The diagnosis of aneurysmal bone cysts and BTs were histopathologically controversial while MRI has significant diagnostic role by showing both cystic and solid portions and fluid levels. Since interventions or simple traumas could cause hemorrhagic areas in the mass, susceptibility artifacts are known to be sensitive but not specific for BTs. Attenuation values of BTs on CT are variable and depend on the internal hemorrhagic, fibrous and cystic components.
Multiple myeloma has to be confirmed with plasma protein electrophoresis and metastasis, chordoma, lymphoma and giant cell tumors could be ruled out with histopathological evaluation. As an initial investigation in a case with multiple lytic bone lesions, plasma calcium, parathormone and phosphorus an vitamin D levels has to be determined. Multiple BTs are seen in cases with parathyroid carcinoma and end stage renal disease due to excess parathormone levels. BTs with numerous lesions are unexpected in primary hyperparathyroidism and a few cases were reported as simulating malignancy [9]. Multiple GCTs may be seen association with Paget disease. The expansile masses without a sclerotic margin in addition to extension to closest articular surfaces were highly suggestive for GCTs. Since GCTs are the second most common type of primary tumor involving the sacrum [10], and the distribution of the lytic masses as the sacrum and distal radius considered GCTs. Spinal involvement of BTs are very rare and thorasic vertebral involvement is most common site while sacral involvement is extremely rare. Multiple BTs in primary hyperparathyroidism in addition to sacral involvement has not been reported [11]. Thus, BTs are great mimickers of GCTs with the clinical, radiological and histopathological aspect depending on the same physiopathological pathway.
Complete resolution of symptoms obtained after one month from resection of the adenoma, and rapid and massive bone formation was observed on MRI. The presentation of hyperparathyroidism is reported to be generally non-specific such as generalised bone and muscle pain, depression, sleep problems, gastroesophageal reflux disease, and decreased concentration but most of the patients with hyperparathyroidism are asymptomatic [12]. The symptoms of radicular low back pain accompanying with hip and elbow joint paint could consider severe osteoporosis and neurologic disorders in cases with hyperparathyroidism could be caused by pathologic fractures or nerve root compressions especially in association with BTs. Although a painful joint movements due to extension of the masses to the articular surface is generally expected from GCTs, BTs would have the similar clinical, radiological and histopathological presentation. Histopathological diagnosis is made on the presence of giant cells with interstitial hemorrhage, microfractures, hemosiderin, and vascularized fibrous tissue with fibroblasts. But any of these findings are not pathognomonic for Brown tumor and giant cell granuloma and giant cell tumor are strong differential diagnosis. Uniformly distributed giant cells without interstitial hemorrhage and fibroblastic stromal cells are expected in giant cell tumors.
BTs with spinal involvement generally require neurosurgical intervention and partial sacrectomy and iliac wing resection is a reported treatment prosedure in a case with sacral and iliac involvement. In the case presented adenoma excision was sufficient although the neurologic symptoms. Surgical excision of adenomas usually provides complete clearance of the lesions, with remineralization of the vertebrae [3]. After evaluation the symptoms and findings such as the bone mineral density, axial involvement associated with neurologic findings and possible pathological fractures; the surgical approach may be limited to adenoma excision in contrary to GCTs or neurosurgical or orthopedic intervention could be added. Since delayed interventions to the spinal lesions could complicate with irreversible neurologic disorders, the patients should be examined and followed up attentively.
In conclusion, a complete regression of BTs could be seen via radiological examination. Both radiologic and symptomatic improvement can be provided with sufficient and effective management on time. BTs are very rare non-touch bone lesions in absence of a pathological fracture. BTs and GCTs should be assessed as differential diagnoses in patients with multiple osteolytic, expansile bone masses. Clinical, radiological, pathological and biochemical correlation in such cases has significant value to achieve a correct diagnosis.
REFERENCES
- Irie T, Mawatari T, Ikemura S, Matsui G, Iguchi T, et al. Brown tumor of the patella caused by primary hyperparathyroidism: a case report. Korean J Radiol. 2015; 16: 613-616. Ref.: https://goo.gl/7unssg
- Pappu R, Jabbour SA, Regianto AM, Reginato AJ. Musculoskeletal manifestations of primary hyperparathyroidism. Clin Rheumatol. 2016; 35: 3081-3087. Ref.: https://goo.gl/5Ls7g6
- Tayfun H, Metin O, Hakan S, Zafer B, Vardar AF. Brown tumor as an unusual but preventable cause of spinal cord compression: case report and review of the literature. Asian J Neurosurg. 2014; 9: 40-44. Ref.: https://goo.gl/fN9iiG
- Tarrass F, Ayad A, Benjelloun M, Anabi A, Ramdani B, et al. Cauda equina compression revealing brown tumor of the spine in a long-term hemodialysis patient. Joint Bone Spine. 2006; 73: 748-750. Ref.: https://goo.gl/ysGBXA
- Van Herden JA, Beahrs OH, Woolner LB. The pathology and surgical management of primary Hyperparathyroidism. Surg Clin North Am. 1977; 57: 557-563. Ref.: https://goo.gl/3Paixh
- Colucci PG, Schweitzer AD, Saab J, Lavi E, Chazen JL. Imaging findings of spinal brown tumors: a rare but important cause of pathologic fracture and spinal cord compression. Clin Imaging. 2016; 40: 865-869. Ref.: https://goo.gl/V1KAWK
- Lim CY, Ong KO. Imaging of musculoskeletal lymphoma. Cancer Imaging. 2013; 13: 448-457. Ref.: https://goo.gl/oPML32
- Murphey MD, Andrews CL, Flemming DJ, Temple HT, Smith WS, et al. From the archives of the AFIP. Primary tumors of the spine: radiologic pathologic correlation. Radiographics. 1996; 16: 1131-1158. Ref.: https://goo.gl/8uN6q8
- Hsu CH, Liew PL, Wang W, Leung TK, Yang KM. Enhanced FDG uptake in brown tumors mimics multiple skeletal metastases in a patient with primary hyperparathyroidism. Acta Radiol. 2008; 49: 949-950. Ref.: https://goo.gl/v1NwL5
- Bloem JL, Reidsma II. Bone and soft tissue tumors of hip and pelvis. Eur J Radiol. 2012; 81: 3793-3801. Ref.: https://goo.gl/iTQDW1
- Fargen KM, Lin CS, Jeung JA, Yachnis AT, Jacob RP, et al. Vertebral brown tumors causing neurologic compromise. World Neurosurg. 2013; 79: 1-6. Ref.: https://goo.gl/o28pwf
- Silverberg SJ, Lewiecki EM, Mosekilde L, Peacock M, Rubin MR. Presentation of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009; 94: 351-365. Ref.: https://goo.gl/n8PE2w